|

"Looking on a forum I frequent, I found a post by Patients Against Lymphoma. It listed many clinical trials around the country. One was a trial at U of M only 50 miles from me. I qualified, and that trial gave me the hope of a better outcome that I was looking for."
~ Tom (WebMagic forum)
Additional background for patients:
Background on study drugs and risks
Questions to ask
.gif) |
Big Picture Questions
|
.gif) |
What is the natural history of my type of lymphoma, and how effective is the standard of care?
|
.gif) |
What are my unique risk factors?
|
.gif) |
What clinical trials are appropriate as therapy for my circumstance - which seem to compare well with the standard of care?
|
Research Advocacy
Our projects:
Background on urgent need to improve accrual
In an era of increasing opportunities to improve outcomes arising from insights into the biology of lymphoma there is a more urgent need to improve accrual!
And related articles on the crisis in clinical research
|
Our primary tools for locating trials and consulting experts:
Rationale for considering trials:
 The good news is that lymphoma and other blood cell cancers are typically sensitive to standard chemotherapy and radiotherapy. Indeed some types of lymphoma are cured with standard approaches. The good news is that lymphoma and other blood cell cancers are typically sensitive to standard chemotherapy and radiotherapy. Indeed some types of lymphoma are cured with standard approaches.
However there remains an urgent need to:
* To increase the cure rate -- and to cure more types of lymphoma
* To improve the duration and quality of responses
* To overcome treatment-resistance
* To decrease the short- and long-term toxicity of treatments overall.
Due to the unparalleled rise in insights into the biology of lymphoma, the opportunities to make progress has never been greater!
See Agents that Target Disease Pathways.
However, getting there depends on our participation in clinical trials.
FDA approvals of new agents are but a first step. Trials are also needed to test the different uses of approved and investigational agents ... such as when given before, with, or just after standard therapies. Studies are also needed to test approved agents in different types of lymphoma and in different settings - such as part of initial treatment.
For these reasons we urge the patient and oncology communities to become well-informed about trials, and for patients to routinely ask there oncologist about trials at consults
Finally, you may want to review Reasons to Consider Clinical Trials based on your unique clinical circumstances. Clinical trials CAN compare very well to regular treatments!
|
Locating Lymphoma Clinical trials
Tools for patients, caregivers, and oncologists
Requiring no registration steps or the disclosure of personal information.
|
|
.gif) |
Lymphoma and CLL  Geographic Search by Geographic Search by
State | World
Or search using MAP of Study Locations
|
|
.gif) |
List All Lymphoma / CLL Trials on ClinicalTrials.gov
|
.gif) |
List New lymphoma / CLL trials on ClinicalTrials.gov since June 2014
|
|
RELATED TOPICS
Four basic goals of therapy
7 Reasons to Consider Trials - based on clinical circumstance
How To Inquire About Clinical Trials
Questions for Your Doctor about Trials
Clinical Questions that Can Be Only Answered by Clinical Trials
Important points about side effects
Conflict of Interest in Medical Decision-making
Project: The Patient-selected Controlled Trials - PCT
A plea for support of our mission and project to advance the
routine and informed consideration of clinical trials 2014-GRANT-concise.pdf
 Here we provide information and tools to assist patients, caregivers, and treating physicians in the routine and informed consideration of clinical trials. Here we provide information and tools to assist patients, caregivers, and treating physicians in the routine and informed consideration of clinical trials.
See Clinical Questions that Can Be Only Answered by Clinical Trials
Our premise is that we can only consider and utilize what we know to exist; and that patients should participate in a study ONLY when the protocol compares well to the standard of care as a treatment decision.
See 7 Reasons to Consider Clinical Trials:
based on our clinical circumstances
Our concern is that the clinical trial referral system is letting us down - is not adequate to make good on the growing promise of clinical science, which is reflected in the large number of lymphoma studies actively seeking participants.
As of December 2010, there are more than
1,300 clinical trials actively recruiting patients.
See All lymphoma/CLL studies, ClinicalTrials.gov
That is, the trial enrollment system has not kept pace with innovations, leading to what we call a "poverty of riches" - an increasing number of targeted agents with a potential to improve our care, in danger of not being adequately tested.
Our Goals
That patients and treating physicians will become active partners in clinical research -
as participants but also as consultants in the design of studies.
.gif) |
To educate patients about the purpose of clinical trials, and how participation can SOMETIMES be in our best interest.
See Clinical Questions that Can Be Only Answered by Clinical Trials
See 7 Circumstances to Consider Trials
|
.gif) |
To notify patients in the support forums about trials of interest, providing also background on the agents and prior study results when possible.
|
.gif) |
To provide doctors and patients with better tools to locate studies that are appropriate to our clinical circumstances and treatment goals.
|
.gif) |
To educate the public about the crisis in clinical research - the need for new policy to make the consideration of trials routine.
See The Terrible impact of low enrollment on clinical research
|
.gif) |
To provide incentives for community physicians to refer patients to appropriate clinical trials.
|
.gif) |
To educate patients about how to inquire about trials with their treating physicians and independent experts.
See Treatment Decision Tree
See How To Inquire About Clinical Trials
|
.gif) |
To educate investigators and sponsors about the importance of providing the rationale for clinical trials protocols as a treatment decision - to help to improve and foster trust in the clinical research system - and guide clinical trial design.
|
.gif) |
To advocate for a effective clinical trial referral process (as part of normal care) and the enhancement of existing tools (ClinicalTrials.gov) so that we can easily locate and consider studies appropriate to our clinical circumstances.
See Proposal for ClinicalTrials.gov (open)
See Our Tools to Help You Find Lymphoma Trials
|
.gif) |
To help patients and caregivers to locate and understand information on the
1) natural history of the disease, 2) the standard of care, and
3) available clinical studies for each lymphoma subtype - noting that an understanding of each is required to achieve informed consent.
|
.gif) |
To provide information and links to resources on travel and lodging, a known obstacle to clinical trial participation.
See Our Travel and Lodging Resource page
|
Consider the goal (or intent) of therapy
.gif) |
To manage the disease: such as to treat a lower-risk lymphoma as-needed with therapy having lower toxicity. This is a common approach to therapy for some types of indolent lymphoma and sometimes for relapsed aggressive lymphoma.
|
.gif) |
To achieve a durable remission: in order to live with no evidence of disease and the frequent need for re-treatment. This is a common intent of therapy for some types of indolent lymphomas.
|
.gif) |
Curative intent: to achieve a possible cure where the disease is eradicated. This is a common intent of therapy for aggressive lymphoma.
For regular treatment the goal can be palliative or best supportive care: such as to relieve symptoms or to address select areas of disease involvement. This is based on meeting immediate needs - similar to the management approach, but based on what is possible instead of what is preferred.
Note: Palliative care can include treatment of the disease (disease management) to relieve disease-related symptoms.
|
The therapeutic misconception:
When considering a clinical trial (as for regular treatment) it's important to inquire about the goal or intent of therapy, which is based in part on the preliminary evidence from completed clinical trials.
As with regular treatment there can be no guarantee that the goal of therapy will be met. The study protocol could be better, the same, or worse than regular treatment. If the answers to the primary study questions were already known there would be no reason to run the study. Still, there are in many cases a hoped-for or anticipated treatment outcome for a study (the study hypothesis).
The goal or intent of therapy is based on the type of lymphoma, the patients age and fitness, and his or her treatment history.
These can change over time as our clinical circumstance changes or as new therapies emerge.
On the so-called therapeutic misconception:
Investigators remind us that the purpose of clinical trials is to answer important clinical or scientific questions -- in order to help future patients and to advance the science.
However, evidence from patient surveys and common sense tells us that patients enroll in studies because of the hope or perception that it can be more effective than regular treatment.
Study enrollment (accrual) can be delayed or fail entirely unless the study protocol has at least a theoretical potential and perception that it can improve outcome compared to regular care.
In our view, the ideal clinical trial achieves both. It's design is such that it can: 1) reliably answer important questions; and 2) it also provides a realistic hope (based on clinical or preclinical science) that the participants might benefit. Indeed it is the duty of treating physicians and IRBs to make sure that the study does not subject the patient to unreasonable risks.
... the most appropriate goal (or intent) of therapy depends on our unique clinical circumstance
We might define cure, as an outcome where the disease never returns, or never returns to a level that is detectable or clinically relevant. For lymphoma, for some types and circumstances, cure is a feasible goal of therapy -- even at an advanced stage. Yet, some approaches to regular or study treatments may have as the intent (or expectation) to manage the lymphoma (perhaps with less toxic therapy) and not to cure.
The urgency to achieve a cure depends on the anticipated clinical course (natural history) of the lymphoma. From necessity, cure is often the intent or goal of therapy for aggressive lymphoma. For indolent lymphomas (today) there is less potential to achieve this goal, but usually less urgency to cure because regular treatments can lead to durable remissions and these lymphoma can also be managed well as needed with lower toxic therapy.
In general the prudent goal of treatment is based on our unique clinical circumstance. For example, an allogeneic stem cell transplant can cure indolent lymphoma, but it also has significant risks, including treatment-related death, and therefore it might not compare favorably to management with lower-risk therapies for a lymphoma that is not considered high risk.
It's important to note that risk and uncertainty are not exclusive to investigational therapies and that clinical trials can sometimes compare favorably to regular approaches. Thus we need to ask questions and to rely on experts to help us with these complex decisions.
So when deciding on a clinical trial (or regular treatment) we and our doctors will consider:
-
The type of lymphoma (indolent, aggressive, and the histology)
-
Our personal risk factors, such as our age and general health
-
The risk of the disease based and its recent behavior
-
The quality or response to prior therapies - including the duration of response
-
The types and number of prior therapies and if there are cumulative side effects
-
The risks and potential benefits of regular treatments - that are standard for our circumstance
-
The strength of the the clinical evidence supporting the study approach
(a few small studies ... or many large studies? Large controlled studies are best.
-
How the reported outcomes fit with the most prudent goal of therapy (and our tolerance for risk) Such as to manage with lower toxicity versus to treat more aggressively with curative intent - or to achieve a durable complete response.
See also Factors that can influence the timing and choice of therapy
The following section describes common clinical circumstances where clinical trials may compare very well to regular treatment:
7 Reasons to Consider Clinical Trials:
based on your clinical circumstances
Patient-Centered Criteria
CONSIDER A TRIAL WHEN:
1. Regular treatment is not yet curative or highly effective;
AND the study protocol has shown (from preliminary evidence*) that it might have the potential to cure, or to improve the outcome - leading to better and longer-lasting responses with less risk and toxicity.
2. Regular treatment is curative, but relapse is common;
AND the study protocol has shown from preliminary evidence that it may improve the cure rate.
3. Regular treatment is curative, but also has significant late toxicities;
AND the study protocol has shown from preliminary evidence that it might be as effective but safer.
4. Standard treatments are not safe for me (because of age/ illness);
AND the study protocol has shown (from preliminary evidence*) that it might have lower toxicity.
5a. Observation is recommended for me, because I have an indolent cancer that does not yet require therapy;
AND the study protocol has low expected toxicity and has shown from preliminary evidence that it might have the potential to delay the need for more toxic treatment.
5b. Management is judged a reasonable approach for a lower-risk lymphoma
AND the study protocol has low expected toxicity and has shown from preliminary evidence that it might have the potential to delay the need for more toxic treatment.
6. The lymphoma is resistant (refractory) to regular therapies;
AND the study drugs work by a novel mechanism – having shown from preliminary evidence that it has the potential to be effective when regular therapies are not.
7. There is no known best treatment for my cancer (a choice is provided);
AND I have no preference and the study protocol will help discover which approved protocol is best for which patient in future.
* The strength of preliminary evidence can range from strong to very weak.
For example, outcome reports from large randomized clinical trials in a population with similar clinical circumstance and the same diagnosis could be considered strong evidence – providing high confidence that the outcomes in the study predict results for others in this circumstance.
On the other hand, small single-arm studies generally provide only modest indications or signals of the potential of a protocol to meet clinical needs; and pre-clinical studies (based on animal models) are considered a starting point only – very preliminary information that the drug or protocol might provide clinical benefit in the future.
On the limitations of regular or standard therapies:
Standard therapies are interventions proven to provide meaningful clinical benefit relative to the natural course of the disease or the disease treated differently.
However, depending on the type of lymphoma, the stage, and patient characteristics, standard therapies can have significant limitations, side effects and risks. That is, not everyone will benefit from it, or the benefit might not be long-lasting, or the risks and side effects may be significant.
.gif) |
PDF version for printing and free distribution PDF
|
.gif) |
|
Understanding your clinical circumstance,
referred to as a treatment setting or indication
Our clinical circumstance is sometimes called a treatment "setting" by investigators, or an "indication" by FDA. New drugs are generally not approved for a cancer, but for an indication --such as previously untreated and advanced stage follicular, or CLL with a specific genetic change (such as 17p).
The population in a study is determined by eligibility criteria, such as the type of lymphoma, previously untreated or relapsed, by age, by performance, by need to treat, by stage and so on. The outcomes of a study report applies only to the eligible population. A response rate of 30% could be encouraging in the treatment refractory setting, but not encouraging in the previously untreated population. So when reviewing clinical trial outcome reports, the eligibility for the study and the characteristics of the patients who took part are important to consider.
Some clinical trial settings are highly specific (narrowly defined); others are more inclusive (with few exclusions). "All comer trials" have few exclusions. Most trials limit enrollment in some ways, such as by one or more of the additional factors listed below.
Increasingly, we will see trials that specify a particular genetic change (a biomarker) as a condition for taking part in the study for a drug thought to target that change. Such studies may require a biopsy to obtain a tumor sample as a condition for enrollment.
The treatment setting can influence the most prudent approach or intent of therapy in clinical trials, but also in
in regular practice.
Common settings (indications) for lymphoma
-
Previously untreated aggressive lymphoma
Additional factors:
Localized or advanced stage?
Bulky or not bulky?
Older or younger in age?
Your performance, fitness, and general health?
Tumor characteristic: cell of origin for DLBCL?
Any prognostic or predictive biomarker available to guide the approach?
-
Previously untreated indolent (slow growing) lymphoma
Additional factors:
FLIPI score?
Localized or widespread stage?
Bulky or not bulky?
Need to treat or no need to treat?
Behavior of lymphoma: progression, stable or slow growing?
Symptomatic / Asymptomatic?
Older or younger in age?
Your performance, fitness, and general health?
Any prognostic or predictive biomarkers to guide approach?
-
Relapsed or Refractory aggressive lymphoma
Additional factors:
Primary refractory or sensitive to prior treatment?
Short or long response to prior therapy?
Localized or advanced stage?
Bulky or not bulky?
You are older or younger in age?
Your performance, fitness, and general health?
Any prognostic or predictive biomarker available to guide approach?
-
Relapsed / Refractory indolent (slow growing) lymphoma
Additional factors:
Primary refractory or sensitive to prior treatment?
Long or short time since last treatment?
Need to treat?
Behavior of lymphoma: progression, stable or slow growing?
Localized or advanced stage?
Bulky or Not Bulky?
Symptomatic or asymptomatic?
Older or younger in age?
Your performance, fitness, and general health?
Any prognostic or predictive biomarkers to guide approach?
Refractory means resistant to the type of treatment received.
Not all relapsed lymphomas (indolent or aggressive) are treatment refractory. A lymphoma that's refractory to one treatment can be sensitive or responsive to another type of treatment.
A biomarker is any test thought to predict high or low risk disease, or a test that potentially predicts a response to a specific type of therapy.
Low or high risk disease is sometimes used to summarize the factors provided above. For example, untreated indolent lymphoma that is localized, non-bulky, and has not progressed over time might be summarized as low risk disease.
Young or older age is relative to the median age of those diagnosed with your type of lymphoma, which is about 65 years for the most common types . Ideally, and to be most reliable, a study population should be similar to the general population with the disease. Typically (unfortunately), trials recruit younger patients for various reasons.
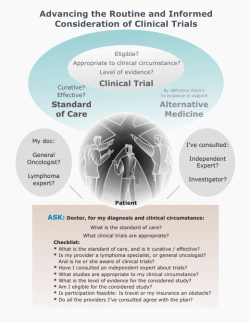
Questions to Ask Your Doctor or Nurse
Is there a study that's right for me (at this center or nearby)?
If so:
.gif) |
What is the purpose of the study?
|
.gif) |
How might I benefit from participating?
|
.gif) |
What are the risks from participating?
|
.gif) |
What were the results of other studies of this treatment?
|
.gif) |
What other treatments could I receive if I don't take part in the study?
|
.gif) |
How do the risks and potential benefits of the study compare with the standard of care for my clinical circumstance?
(Circumstance? Our diagnosis, treatment history, risk factors.)
|
If I join the study:
.gif) |
How am I protected from possible adverse events?
|
.gif) |
What tests are in the study, and how often are they given?
|
.gif) |
Can I decide to stop participating at any time? (Yes, you can)
And if so, will it affect the care I receive? (No, it will not)
|
.gif) |
Will I continue to see my current doctor?
|
.gif) |
How will participating effect daily activities? Will I still be able to work or go to school?
|
.gif) |
Will I have to be in the hospital? How many days?
|
.gif) |
What are the possible risks or side effects for me?
|
.gif) |
Will my insurance cover procedures and tests?
If not, will the drug sponsor cover it?
|
.gif) |
Will there be extra costs because of the study?
|
.gif) |
How will I be checked after the study, and how often?
|
.gif) |
How long will the study last and how long will I be tested?
|
.gif) |
Will I be made aware of the results?
|
Important Points About Side Effects:
Side effects and uncertainty about them are not unique to clinical trials.
Here are important points about side effects that may apply to studies.
.gif) |
The study doctors do not know who will or will not have side effects. |
.gif) |
Some side effects may go away soon, some may last a long time, or some may never go away. |
.gif) |
Some side effects may interfere with your ability to have children. |
.gif) |
Some side effects may be serious and may even result in death. |
Here are important points about how you and the study doctor can make side effects less of a problem:
.gif) |
Tell the study doctor if you notice or feel anything different so they can see if you are having a side effect. |
.gif) |
The study doctor may be able to treat some side effects. |
.gif) |
The study doctor may discontinue or adjust the dose of the treatment to try to reduce side effects.
|
The Terrible impact of low enrollment on clinical progress
One in Five Cancer Clinical Trials Is Published:
A Terrible Symptom—What's the Diagnosis?
http://theoncologist.alphamedpress.org/cgi/content/full/13/9/923
.gif) |
"half of the unpublished trials have failed to accrue and reach endpoints;
|
.gif) |
Nearly 60% of trials opened for 5 years had fewer than five patients enrolled at each site, and, for >20% of studies, not a single subject had been accrued.
|
.gif) |
of all NCI phase I, II, and III trials opened and closed between 2000 and 2007,
only 50%–60% achieved minimal stated accrual goals.
|
.gif) |
So, while perhaps only one in five cancer clinical trials is ever published,
of those unpublished, a significant percentage probably died for lack of accrual.
|
Related Reports
Active and Completed Surveys
PATIENT SURVEY (completed)
To identify obstacles to participating in clinical trials, and find remedies:
Interests, Attitudes, and Participation in Clinical Trials Among Lymphoma Patients With Online Access
.gif) |
Patient Survey on attitudes and participation in trials Web-based (closed)
|
.gif) |
Poster exhibit PDF (accepted L&M Conference)
.gif) |
Narrative of Poster Exhibit PDF
|
|
.gif) |
ASCO 2009 JCO
|
Discussion: Patient issues and perceptions regarding randomization, study risk, eligibility, and tests and procedures suggest an opportunity to improve enrollment in clinical trials by focusing on these aspects of study design, specifically, attending to the rationale of the protocol as a treatment decision – having the potential to optimally meet the clinical needs and treatment goals of the participants, in addition to answering important clinical questions.
ONCOLOGIST SURVEY (open)
To identify obstacles to the routine consideration of clinical trials:
.gif) |
Confidential CLINICAL TRIAL SURVEY
For Oncologists Treating Lymphoma / CLL Patients PDF
ACTION: Patients: Please print the survey and bring to your next consult!
|
.gif) |
Raw data (preliminary n = 51)
|
.gif) |
Raw data PDF
|
.gif) |
Most commonly cited obstacles so far (n=51) Chart PDF
|
.gif) |
Most significant obstacles so far Chart PDF
|
CLINICALTRIALS.GOV - in need of an upgrade! (open)
To make ClinicalTrials.gov searchable by clinical circumstances and goals:
.gif) |
Our proposal to NIH ClinicalTrials.gov group PDF
This proposal has been received by NIH and forwarded to the PDQ group.
A teleconference has been offered to discuss.
|
Related Perspectives
.gif) |
Free brochure on this project for printing and distribution:
The Urgent Need to Increase Participation in Clinical Trials PDF
|
.gif) |
Presentation on participation issues to industry and investigators:
Harmonizing Research Goals with Meeting the Clinical Needs
and Treatment Goals of the Participants PDF
|
.gif) |
Questions that Can Be Only Answered by Clinical Trials PDF
|
A "poverty of riches"
There's an abundance of rationally designed promising new agents, which need to be tested as single agents, but also with existing protocols. However, the pool of patients considering clinical trials remains very low, therefore studies failing to reach enrollment goals are increasing as the number of promising agents increase.
It's as if you have a small pond with 100 fish, and a growing number of fisherman (once 10, now 20) testing new lures. Each contestant making it more difficult to know which if any is truly effective, useful, or best for which type of fish.
A marker for this was the prevalence of N=6, N=10, N=20 in presented clinical data. Such small populations, while customary in early phase studies, contributing to missed signals of benefit and risk. Low enrollment and delays in enrollment causing sponsors to reconsider if the investment is worth while - could ever lead to marketing approval.
Conclusion: We urgently need to increase the pool of patients considering clinical trials by providing new tools and clinical practice policies.
Overcoming Obstacles to Participation in Clinical trials:
DRAFT - requires revision
.gif) |
Our proposal to Enhance ClinicalTrials.gov - make searchable by clinical circumstances PDF
|
.gif) |
Brochure: The Urgent Need to Increase Participation in Clinical Trials PDF
|
.gif) |
Patient attitudes and interests in Trials - Our Poster Exhibit PDF
|
.gif) |
Questions that Can Be Only Answered by Clinical Trials PDF
|
.gif) |
Harmonize research objectives with meeting clinical needs and treatment goals
If patients fail to sign on in adequate numbers, the assessment of the therapy will not be made, no matter how well the study is designed from the point of view of regulators and drug sponsors.
Biospecimen-based studies are needed to address patient and disease heterogeneity.
Accounting for patient and disease variables could reduce risk (real and perceived) of study participation, making participation in a trial more attractive to patients than standard medicine.
|
Action: Encourage sponsors to consult informed patients, such as scientists with the disease, when designing clinical trials.
Action: Visualize the study protocol from the patient’s point of view – with an appreciation that patients have one life to experiment with.
.gif) |
Does the protocol compare favorably to other options in this setting?
|
.gif) |
Does it unnecessarily include tests that patients fear, such as bone marrow biopsies?
|
.gif) |
Do the side effects preclude the use of treatments that may be needed later?
|
.gif) |
Does the protocol include quality of life endpoints?
|
.gif) |
Is there genuine uncertainty about which treatment arm in a randomized trial is best?
|
.gif) |
Can you disclose outcome data as it develops, to foster trust?
|
.gif) |
Are predictive tests used to identify who is likely to benefit, such as gene profiling? *
|
“Given the number of articles that describe the correlative and predictive usefulness of array-based molecular classifications, especially in leukemias, these outcomes no longer elicit surprise. But perhaps they should — not only because of their clinical usefulness, as outlined in the editorial by Grimwade and Haferlach (pages 1676–1678), but also because of the rapid pace at which expression genomics is changing the conduct of clinical research.”
NEJM ~ Vol. 350:1595-1597, April 15, 2004 No 16
Also see Patient perspectives on clinical trial design
.gif) |
Carry out the blueprint for the National Biospecimen Network
in order to conduct more efficient and humane drug development and evaluations:
.gif) |
Standardized collection of biospecimens;
|
.gif) |
Find novel targets using microarrays;
|
.gif) |
Refine diagnosis and identify high- and low-risk disease;
|
.gif) |
Find clinically useful biomarkers by identifying correlations between gene expression profiles and responders;
|
.gif) |
Select the appropriate patients for targeted-phase studies.
The result is that smaller studies will be needed to achieve statistically significant results, providing relief from the competition for patients.
Fewer patients will be subjected to the toxicity of drugs that cannot help them.
The new favorable circumstances – replacing trial and error – will cause increased interest in trials and cancer research among patients, investigators, and commercial entities...
See background on National Biospecimen Network
|
|
.gif) |
Encourage “trial talk” between the patients and treating physicians
.gif) |
Action: Help patients and physicians to locate appropriate studies by:
.gif) |
using the lymphoma-specific queries of ClinicalTrials.gov on our website;
|
.gif) |
advocating for patient-friendly descriptions of clinical trial protocols and links to related data;
|
.gif) |
encouraging sponsors to list studies in ClinicalTrials.gov as mandated by FDA.
NOTE: We consult with patients and experts to identify notable clinical trials for lymphoma,
and invite drug sponsors to inquire about adding protocols to our list.
See Clinical Trials of Interest for Lymphoma.
|
|
.gif) |
Action: Provide community physicians with incentives for referring patients to clinical trials.
|
.gif) |
Action: Involve community physicians in the administration of clinical trial protocols.
|
.gif) |
Action: Educate patients about the true risks of the disease, the potential of investigational treatments, and the limitations of standard therapies. See, for example About Lymphoma
|
.gif) |
Action: Encourage patients to consult lymphoma experts to get objective opinions about investigational therapies. See Doctors page.
|
.gif) |
Action: Advise patients to have the experts they consult contact their treating physicians in order to reach a consensus on what treatment protocol is best for them.
|
.gif) |
Action: Help patients to review consent forms, contact investigators, and complete applications.
|
.gif) |
Action: Inform patients on how to better evaluate medical claims and data.
|
|
.gif) |
Create innovative trial designs with the potential to make a positive difference in patients' lives
The time to get creative is early in the course of the disease, when the patient has better immune competence, less tumor burden, and has had fewer exposures to toxic therapies.
.gif) |
Action: Develop front-line protocols, combining aggressive approaches in combination and/or in sequences with biological and immune-based therapies, that provide the potential for curing or extending survival in difficult to treat lymphomas.
|
.gif) |
Action: Develop front-line protocols for indolent lymphomas that combine or sequence low toxic target biotherapies and immune-based therapies with the goal of managing the disease with minimal toxicity.
NOTE: Without publicity, a frontline idiotype vaccine study completed accrual in two weeks.
|
.gif) |
Action: Develop protocols that induce active immunity against tumor antigens, and that may overcome ways that tumors escape or suppress immunity.
|
.gif) |
Action: Reduce reliance on quick response agents when the responses are not durable and the side effects, such as myelosuppression, contribute to complications and limit future options.
|
.gif) |
Action: Develop protocols for refractory end-stage disease that can extend survival while improving quality of life.
|
|
Related Resources & News
 Topic Search: Clinical Equipoise (genuine uncertainty in randomized studies) Topic Search: Clinical Equipoise (genuine uncertainty in randomized studies)
.gif) |
The Urgent Need to Increase Participation in Clinical Trials PDF
|
.gif) |
Cancer Trial Participants Rarely Told of Results Medscape (free login req.)
"That may be changing, however, as patient advocates and some clinical researchers assert that offering results recognizes patients as partners in the research process, according to Dr. Partridge."
|
.gif) |
|
.gif) |
|
.gif) |
|
.gif) |
|
.gif) |
|
.gif) |
|
.gif) |
|
.gif) |
Review the article: Experimental Therapy, End-of-Life Care Can Coexist
Tue May 21, 7:12 PM ET HealthScoutNews
|
.gif) |
|
.gif) |
Obstacles to the accrual of patients to clinical trials in the community setting. Winn RJ.
Semin Oncol. 1994 Aug;21(4 Suppl 7):112-7.
|
.gif) |
Things you can do to get your health insurance to cover a clinical trial cancer.gov ... still an issue until 2014, when the Health Care Reform measure kicks in.
|
.gif) |
Understanding the attitudes of the elderly towards enrolment into cancer clinical trials http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1382233/?tool=pmcentrez
"The optimal cancer treatment for an older population is largely unknown because of the low numbers of elderly patients accrued into clinical trials. ...
Cancer is a disease of the elderly with 60 % of cancers occurring in those over the age of 65 years [1]. As our population ages it will become increasingly critical to optimise treatment in older patients to ensure both good quality of life for this group and the best allocation of medical resources."
|
.gif) |
Barriers in phase I cancer clinical trials referrals and enrollment: five-year experience at the Princess Margaret Hospital ncbi.nlm.nih.gov
|
|