|
Search Site Guidelines at Diagnosis | About Clinical Trials How to Help! |
|
|
Patients Against Lymphoma |
|
|
|
|
What is a Drug?
Is the drug active? Is it effective?
|
||||||||||||||||||||||||||||||||||||||||||||
|
Aspirin, for example, can help to reduce the sensation of pain by inhibiting inflammatory enzymes in our body. |
|
|
Other painkillers put locks on neural receptors to reduce the sensation of pain. |
|
|
Antibiotics, such as the well-known penicillins, work by killing bacteria. |
|
|
Chemotherapy agents damage rapidly dividing cells, causing the cells |
Before aspirin there was willow bark. Opium and cocaine relieved pain. Early scientists extracted and purified the active ingredients from such early natural compounds. Clever organic chemists delighted in fragmenting these molecules in order to find their structures.
Aspirin and penicillin have been around for a long time, but the drugs that stop and kill cancer cells are among the newest drugs being discovered and fine-tuned.
|
How do scientists develop them? |
|
|
How do we know what happens when a drug is received in the body, |
Briefly, the investigation is done in phases: preclinical, clinical (human testing), and regulatory assessment.
The first task in in the preclinical phase is to find by screening or design a promising compound and then to determine how much of the drug is needed to do the job. The necessary concentration is determined in preclinical experiments involving cell cultures as in the well-known Petrie dish, or with animals.
(NOTE: This phase is essential to credible drug research. Sometimes herbal products are inappropriately hyped as cancer treatments based on cell culture experiments, without accounting for the concentration needed in the blood to achieve the cell-culture effect, or if that concentration would be toxic.)
Increasingly for cancer and other diseases new compounds are designed to bind to parts of cell that are driving cell survival or proliferation. These abnormal working parts of the cell are called disease pathways.
See also Agents that target disease pathways
In the body the drug interacts with, binds to, or disrupts, some process underlying the disease. Targets of the compound can be cell membranes; enzymes, structures or carriers - all proteins - or any one of the cellular chemicals or processes that have been hijacked to keep the disease going.
The fit between a drug and a body molecule or diseased cell structure is known as affinity.
One important goal of therapy related to affinity is specificity ... that the compound binds as exclusively as possible to the target of treatment and minimally impairs normal processes.
The interaction between drug and disease is known as mechanism of action - how the drug works.
However, even well-targeted drugs, such as the cancer drug Gleevec, can have off-target effects: significant side effects, emphasizing the need for caution in the testing of new drugs in human subjects, particularly classes of drugs that work by new mechanisms of action or even when approved agents are combined for the first time.
A relatively new drug target is found on the surface of cancer cells. These are molecular binding sites. One binding site, known as CD20, is targeted by the drug Rituxan. Here a mouse/man-made antibody binds to the CD20 receptor which can cause the cell to self destruct, or leads to killing of the tumor cell by immune cells.
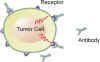
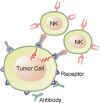
Click antibody engaging b-cell tumor images to enlargeThe field of identifying new potential drug targets is accelerating. Drug targets may be within the cell, such proteins or genes that prevent cell death, on the cell surface as described above, or the target may be other "normal" cells that contribute to the survival and expansion of the malignant cells in the tumor microenvironment.
Many chemotherapy drugs exploit the overt behavior of cancerous cells - rapid cell division. The dividing cell more readily takes up the drug, which leads to damage of its DNA (the vital information that determine cell behavior and functions) which is more vulnerable during cell division. ...
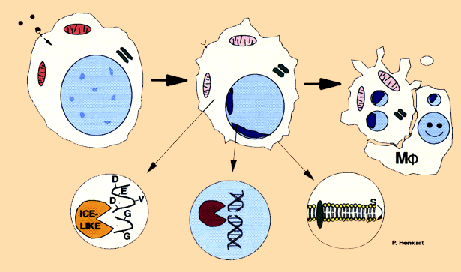
Source: http://www.nih.gov/sigs/aig/
... The cell, detecting the damage to its DNA , self destructs in a process called apoptosis, similar to when diminishing light triggers leaves to fall from trees.
Abnormal cells, being living parts of the body, can adapt to drugs leading to drug resistance. Here getting the dose right and the rationale combination of drugs can minimize the ability of the cells to adapt and survive. Combining drugs can have an ad
There can be an additive or synergistic effects when combing drugs agents. Also it's possible that one active agent can work against the mechanism of another active agent (as an agonist) - such as when the second agent causes cell cycle arrest, which is needed for first agent to work.
Additive: 1+1 equals 2
Synergistic: 1+1 equals 3
Agonistic: 1+1 is less than 2Researches will also study the mechanisms of resistance. To do this they will need tumor sample from the participants of studies - samples before and after resistance to identify what pathways are turned on in the cells that survive the targeted agent - that may also be targeted. ... As was done with Gleevec.
The dose differentiates a remedy and a poison. ~ Paracelsus
That a compound is active is just the starting point in the drug development process.
The agent might be highly toxic at the concentration needed be active in a test tube (In vitro). Or it may not be absorbed well if taken orally, or it may be cleared too quickly to have a meaningful treatment effect in the body (in vivo).
Thus, pharmacokinetics (PK) is an essential part of new drug development and assessment in the clinical phase. It's the study of what your body does to a drug. The initial PK research is carried out on animals and then ever-so-slowly and carefully in humans. See also Wikipedia.org
How long the drug remains in the bloodstream, and at what concentration, are vital to the safety and effectiveness of drug, which are determined by
|
Absorption, |
|
|
Distribution, |
|
|
Metabolism and |
|
|
Excretion |
The organs that have a major impact on ADME are the liver and the kidneys and therefore liver and kidney function are monitored very closely when new drugs are first administered in humans.
Individual differences in ADME can result in faster or slower clearance of the drug from the body.. Differences in clearance rates can affect the course of treatment and severity of side effects.
(See also the side bar on HETEROGENEITY.)
If the drug remains in the blood too long it can increase side effects. Conversely, a drug that's excreted or cleared too rapidly will not be around long enough, or at the proper concentration, to do its job well.
Moreover, interaction with other drugs or herbs can affect how drugs are absorbed or cleared from the body.Yet another vital aspect of the drug development and testing is the called pharmacodynamics, or PD, which is the study of the effects of drugs on the body or on disease processes within the body and the mechanisms of drug action and the relationship between drug concentration and effect. See also Wikipedia.org
The objective of PD studies is to identify the therapeutic window: the dose needed to achieve sufficient levels of the agent in the blood at acceptable toxicity.
The larger the therapeutic window the more likely the drug can be administered in a safe and effective protocol.
If the new drugs shows reasonable safety and activity in early phases of clinical trials, it is then tested against approved therapies in large randomized controlled clinical trials. The goal of phase III studies is to objectively determine the safety and efficacy in a way that minimizes bias.
Notably, failures outnumber successes in the drug development process. Only 1 in 5,000 new compounds evaluated in the preclinical stage makes it to the clinic ... and about 1 in 5 new therapies that reach phase III clinical testing.
The regulatory evaluation of the data submitted by the sponsor for marketing approval does not take very long ... about 6 to 9 months; while the preclinical and clinical testing phase can take ten years or longer. The cost to the sponsor can exceed 1 billion dollars.
Because the financial risks of cancer drug development are high and most new drugs fail to win approval, incentives (primarily in the form of marketing exclusivity) are granted to new drugs that win approval.
Notes on the dose
|
|
Introduction to Pharmacokinetics and Pharmacodynamics ashp.org |
|
Patents wikipedia.org |
|
Treatment mechanisms | Treatment types |