|
Comment on
BIOVEST PACE-vaccine study
In the BIOVEST study patient-specific idiotype vaccine was tested in patients who achieved a Complete Response (CR) after PACE chemotherapy, compared to a placebo vaccine
The study never completed enrollment, which is required to achieve statistical significance* -- to exclude the influence of chance - in this case the normal variation in outcomes to the same pretreatment.
*NOTE: If you did a randomized study and gave each group identical placebo (saline) after the same chemo, by chance (based on differences in the patients and underlying disease) one group would do better than the other. So even for a large randomized study the magnitude of the difference in outcome must be sufficient to exclude the effect of chance - for example, that one group had disease more sensitive to chemo than the other.
So in the BIOVEST study full enrollment was never completed, because of the toxicity of the PACE chemo and the low CR rate, required to be eligible to receive the vaccine.
So for the patients who achieved a CR and received vaccine the Progression Free Survival (PFS) favored the vaccine arm in the BIOVEST study, but was judged by the sponsor (and its advisors) to be of marginal statistical significance ... not strong enough for the sponsor to bring the data before FDA to apply for marketing approval, but also suggesting that the vaccine might be active and effective.
Since the CR rate with the highly toxic PACE was low, many participants were not in the analysis .... In other words: a *big percentage* of patients who entered the study did not respond well enough to PACE to receive vaccine or the control vaccine.
NOTE: You get but one chance to have first primary therapy for a lymphoma, probably this is your best chance to achieve a durable remission, so the low CR rate for PACE is potentially a liability for the majority of the participants - in the intent-to-treat population.
Since the standard of care for induction therapy is now CVP-R or CHOP-R, B-R, or RIT ... the sponsor would have to begin again to test the vaccine after use of one these induction protocols.
However, since Rituxan and RIT depletes normal b-cells (antibody producing cells), it's not known if the idiotype vaccines (possibly mediated by antibody against idiotype) will be active after R-based chemo or RIT.
I truly have no personal opinion or expectation regarding the promise of vaccines, or any investigational agent. I've come to realize they are called investigational for a reason.
So far, the Genitope and Favrille studies were good controlled studies (fair tests) which showed no difference (no effect for vaccine).
The Biovest study shows a possibility of activity and benefit, but falls short of proof because of the low enrollment, and "the possibility of imbalanced arms." [Meaning the risk factors, such as disease status, age, etc., may have been higher in one arm.]
"This third trial differed from the other two by having a greater proportion of patients who were in complete remission at the time of vaccination. If validated, this result might provide additional evidence for the value of vaccination in the minimal disease state." ~ Brody, Kohrt, Marabelle, Levy
But even if the Biovest study completed enrollment it would not have informed us if PACE followed by vaccine is better than Rituxan-based chemo alone... which brings us to Dr. Levy's comment:
... in the time since patients were recruited, rituximab has added a powerful advantage in improving the treatment of follicular lymphoma.
There still could be a role for vaccination, but there need to be trials in which the vaccine Is given before and/or after treatment with chemotherapy plus rituximab, he said.
How much did the study miss by in terms of enrollment?
To achieve statistical significance
the study required about 563 pts for 2:1 randomization in order to randomize 375 pts to either arm *
250 pts in the vaccine arm
124 pts in the control arm …
* Because a CR was needed to be randomized, the study had to enroll more than 375 patients because not everyone gets a CR.
The study managed to randomize a fraction of the needed patients - 117 pts, of these:
76 pts were randomized to the vaccine arm
(far short of the goal of 250)
41 pts in the control arm
source: Follicular Lymphoma: Experimental Vaccine Extends Disease-Free Survival
oncology-times.com
~ KarlS (independent commentary)
CLINICAL TRIALS
|
Treatments > Vaccines
Last update: 08/11/2016
|
|
TOPICS
Comment on BIOVEST Study
About | Clinical Trials | Enhancing vaccines?
Recent Reports | Background Articles | Outcome Data | Comment on Biovest Data
 TOPIC SEARCH: TOPIC SEARCH:
PubMed: Treatment | Review | Dendritic | Idiotype | DNA type | Heat Shock type
By site: ASCO | ASH | ClinicalTrials.gov | Medscape | Web
By authors: Bendandi | Levy | Timmerman
|
|
The goal of vaccine therapy is to teach the immune system to recognize and attack tumor cells.
Background: There is a molecule on b-cells that is specific to the shape of a bacteria, or virus, or ... anything foreign to the body, which is called an antigen. This receptor fits like a key to a lock in order to bind the antigen.
When a b-cell becomes malignant, all of the descendents of the cell inherit the same b-cell receptor, which, unlike cd20, is expressed only on the tumor cells
To make the vaccine, a portion of this receptor, called the idiotype was isolated from the tumor sample and then replicated by different techniques. The idiotype protein was then coupled with KLH, a highly immunogenic substance derived from a shellfish.
So the vaccine was part tumor antigen and part shellfish: Id-KLH. The idiotype for each patient's FL is unique to the patient's tumor - hence it is called a customized or personalized vaccine.
It was an ingenious concept: to try to teach the immune system to consider the idiotype to be like KLH, something to reject. The hope was that the immune system would create antibodies against the idiotype - helping to get rid of any tumor cells that survived chemo.
Unfortunately, when tested in two large randomized studies (Genitope and Favrille) the control group did as well as those who had the custom vaccine, which was given as injections under the skin following induction therapy (chemo in one, rituxan in the other) along with an immune stimulant.
The Biovest version of the idiotype vaccine - the first to start testing and last to "finish" -- never completed enrollment because the induction therapy (PACE) was too toxic. It showed a better result for those receiving the vaccine, but it was not a statistically significant difference - could well have been coincidental - the number of patients in the study was not large enough to provide certainty.
See right column for detailed comments
NOTE: An immune response as a result of vaccination with idiotype vaccine is not the same as a response to treatment - it's an indication that your body created antibodies against the idiotype component of the vaccine. Whether that translates into immune cells killing of tumor cells is not known.
So the phase III study was designed to find this out by measuring time to progression in the two groups. If the vaccine arm had a significantly longer time to progression than the control group we could have concluded that
vaccine - by helping the body to recognize a tumor antigen -- was an indirect causal factor in the delay of recurrence.
The vaccine itself does not interact with the tumor; the hope is that the antibodies created in response to the vaccine will bind to the idiotype on the tumor cells and lead to their death (similar to how rituxan works, but the binding site is the idiotype instead of cd20.
How could it be that an immune response (antibodies against idiotype) would not make a clinical difference? As noted by Suzanne, detecting a response may not tell us about the strength of the response: the amount of anti-idiotype antibody that your body created in response to vaccine. It might not be enough to affect a significant proportion of the tumor cells. Also, even if there is enough antibody it doesn't mean it will lead to killing of tumor cells. Consider that not everybody that receive rituxan (an antibody) will have tumor regressions. Perhaps the binding of the anti-idiotype antibodies is weak or has an imperfect affinity to the receptor on the tumor, or the tumor is resistant to death from this effect
....
So the idiotype vaccine is injected into the skin. The immune system takes it up, and hopefully creates antibodies against the idiotype in the vaccine, which should correspond to the idiotype on the tumor. The antibodies created by the body in reaction to the vaccine can be detected in a test, and if so would be called an immune response to the idiotype of the
vaccine. These antibodies may or may be a good fit to the tumor, may or may not be in sufficient quantity to have an effect; and may or may not lead to tumor regressions.
The injection of the vaccine (Id+KLH) is followed by an injection of GM-CSF in the same location. GM-CSF is a colony stimulating factor that can increase the number of immune cells and help specialized immune cells, called dendritic cells, to mature. The job of dendritic cells is to present foreign antigens to other types of immune cells to help complete the immune "education" process.
The Idiotype vaccine has been tested mainly as a follow up treatment to chemotherapy - so-called consolidation therapy. After a rest period the vaccine is given with the goal of teaching the immune system to kill off any residual cells that have survived the chemotherapy. One pilot study (that accrued patients very quickly) is testing the efficacy of the Id vaccine on untreated patients without the use of any chemotherapy. Another pilot study (by Favrille) tested the feasibility of using the Id vaccine to regress tumors in previously treated patients without pretreatment with chemotherapy. A phase III controlled study is also testing the ability of the Idiotype vaccine to improve response to Rituxan therapy.
It's important to realize that this approach is unproven in lymphoma and that the many experts do not believe it likely that this type of treatment could benefit patients with bulky or progressing disease.
Passive versus Active Immunotherapy.
Passive immunotherapy, such as Rituxan, involves infusion of antibody that induces an immune response to attack the cells it binds to. The infused antibody has a half life and thus, a limited duration of clinical activity. In theory, idiotype vaccines can induce long lasting active immunity, in which the body produces antibody against the tumor antigen as a result of recognition of this antigen being abnormal (foreign). Thus, the immune response involves memory and can be active for a very long time ... It can be activated by the presence of tumor expressing the idiotype antigen and not dependent on infusions of antibody. Another potential advantage of idiotype vaccine is that it activates immunity against tumor cells only. Normal cells will not be harmed.
Finally, it's important to note that only the results of controlled clinical trials can inform us just how effective active immunotherapy with idiotype vaccines will be, and for how many patients. Detecting an immune response to the idiotype antigen does not guarantee the patient will receive clinical benefit. The malignant cells may have ways to evade or suppress an immune response, for example. By November 2007 the results of Genitope study should provide the earliest indication about the efficacy for this version of the idiotype vaccine. The different idiotype vaccine may not all be equivalent in potential, and the choice of conditioning therapy could also influence the outcomes significantly.
|
The antibodies are magic bullets which find their targets by themselves, so as to strike at the parasites as hard and the body cells as lightly as possible.
"The Greek root word for "unique" is idiotype, and that is the term used to describe the specific shape and structure of antibody molecules, which sit on the surface of B cells like receptors, tasting and testing scraps of protein in the bloodstream."
- Stephen S. Hall, A Commotion In The Blood
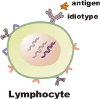
B-cell with Idiotype protein.
Click to enlarge
|
.gif) |
Vaccine treatment for NHL on ClinicalTrials.gov
|
.gif) |
Lymphoma Vaccine Enters Large-Scale Clinical Trials
|
.gif) |
Antigenics HSP Vaccine: clinical trial - MDACC
|
.gif) |
Clinical Benefit of Idiotype Vaccines: Too Many Trials for a Clever Demonstration? RRCT.pdf
Commentary, Maurizio Bendandi1,2,3*
|
NEWS & RESOURCES
.gif) |
2016; Cancer Vaccines: Are They the Wave of the Future? : Oncology Times http://bit.ly/2blp13O
Types and challenges described
|
.gif) |
Recognition of live phosphatidylserine-labeled tumor cells by dendritic cells: a novel approach to immunotherapy of skin cancer. Shurin MR, Potapovich AI, Tyurina YY, Tourkova IL, Shurin GV, Kagan VE. Cancer Res. 2009 Mar 15;69(6):2487-96. Epub 2009 Mar 10. PMID: 19276376
Dendritic cells (DC) loaded with tumor antigens from apoptotic/necrotic tumor cells are commonly used as vaccines for cancer therapy. However, the use of dead tumor cells may cause both tolerance and immunity, making the effect of vaccination unpredictable. To deliver live tumor "cargoes" into DC, we developed a new approach based on the "labeling" of tumors with a phospholipid "eat-me" signal, phosphatidylserine.
|
.gif) |
Oct 2008: Biovest Announces BiovaxID® Anti-Cancer Vaccine Prolongs Cancer-Free Survival by 44%
that the median duration of complete remission in the BiovaxID arm of the study was 44.2 months which is clinically and statistically significant compared to the control arm, median duration of cancer-free survival of 30.6 months. BiovaxID prolonged the cancer-free survival by 13.6 months or 44% (p-value = 0.045; HR = 1.6) with a median follow up of 56.6 months (range 12.6 to 89.3 months). Press release
|
.gif) |
Sept 2008 - Pilot vaccine report: Vaccination with autologous tumor-loaded dendritic cells induces clinical and immunological responses in indolent B cell lymphoma patients with relapsed and measurable disease: a pilot study. Blood. 2008 Sep 22. PMID: 18809757
Collectively these results [single arm study, N= 18] demonstrate that vaccination with tumor-loaded DCs may induce both T and B cell responses and produces clinical benefits in indolent NHL patients with measurable disease.
|
.gif) |
Basic research: Follicular lymphoma B cells induce the conversion of conventional CD4(+) T cells to T-regulatory cells. Int J Cancer. 2008 Sep 23. PMID: 18814264
Our study provides evidence for a tumor-specific mechanism by which FL tumor cells promote immune escape through the induction of Treg.
|
.gif) |
Outcomes: Summary of MyVax Personalized Immunotherapy Phase 3 Clinical Trial Results media.corporate-ir.net pdf
"Genitope believes that the failure of its MyVax Phase 3 clinical trial to meet its primary endpoint was due to unrecognized activity in the non-specific immunotherapy."
|
.gif) |
Bad news: Genitope halts MyVax work, examines future bizjournals.com
... said it will suspend work on its cornerstone personalized cancer vaccine, MyVax, for follicular non-Hodgkin's lymphoma.
Also see our commentary
|
.gif) |
Genitope Corporation Reports Initial Results of Phase 3 Clinical Trial of MyVax(R) Personalized Immunotherapy Dec 2007
|
.gif) |
Background: Genetic vaccines - Google books
|
.gif) |
Preclinical: A novel proteoliposomal vaccine elicits potent antitumor immunity in mice.
Blood. 2007 Jun 15;109(12):5407-10. Epub 2007 Mar 9. PMID: 17351111
|
.gif) |
Opinion: The Disease is the Remedy time.com
"In addition to showing that the vaccine can prolong remission, Bendandi's trial attempted to solve the long-standing problem of quantifying the results of custom-made treatments. "
|
.gif) |
ASH 2003 Update: Cellular Therapies and Vaccines for Hematologic Malignancies Conference Coverage ~ cancerconsultants.com
|
.gif) |
Harvesting and handling tissue in preparation for vaccines PAL
|
.gif) |
Background: Cancer vaccines fact sheet cancer.gov
|
.gif) |
Ronald Levy MD, David Fisher MD
.gif) |
Testing Vaccines for Non-Hodgkin's Lymphoma
Healthology (Nov 2003)
|
|
.gif) |
Stimulation of cytotoxic T cells against idiotype immunoglobulin of malignant lymphoma with protein-pulsed or idiotype-transduced dendritic cells
bloodjournal.org/cgi/content/full/95/4/1342
|
.gif) |
Treating cancer with vaccines cancer.gov
|
.gif) |
Immune-based therapies PAL
|
.gif) |
Cancer vaccine notes PAL
|
|