|
Side Effects > Cardiac (Heart)
Last update: 09/30/2014
|
 TOPIC SEARCH: TOPIC SEARCH:
PubMed - anthracyclines | PubMed - general | Scholar Search
Resources
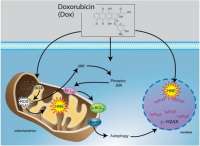 Anthracyclines (such as doxorubicin) are very important (effective) agents for the treatment of lymphomas, however the use of anthracyclines increases the risk of damage to the heart (cardio toxicity. The risk of cardio toxicity is greater for the elderly and pediatric patients, and in persons with preexisting heart issues. The risk is also related to the cumulative dose of the anthracycline drug, and possibly the rate of administration - how fast it is given. Anthracyclines (such as doxorubicin) are very important (effective) agents for the treatment of lymphomas, however the use of anthracyclines increases the risk of damage to the heart (cardio toxicity. The risk of cardio toxicity is greater for the elderly and pediatric patients, and in persons with preexisting heart issues. The risk is also related to the cumulative dose of the anthracycline drug, and possibly the rate of administration - how fast it is given.
“The incidence of congestive heart failure secondary to doxorubicin-induced cardiomyopathy depends on the cumulative dose of the drug.
At total doses of less than 400 mg/m2 body surface area, the incidence of congestive heart failure is 0.14%; … this incidence increases to 7% at a dose of 550 mg/m2 body surface area and to 18% at a dose of 700 mg/m2 body surface area [6] (Figure 1).
The rapid increase in clinical toxicity at doses greater than 550 mg/m2 body surface area has made the 550-mg dose the popular empiric limiting dose for doxorubicin-induced cardiotoxicity. “ 11
Overview Article:
Cardiotoxicity of chemotherapeutic agents:
incidence, treatment and prevention. ncbi.nlm.nih.gov
Cytostatic antibiotics of the anthracycline class are the best known of the chemotherapeutic agents that cause cardiotoxicity.
Alkylating agents such as cyclophosphamide, ifosfamide, cisplatin, carmustine, busulfan, chlormethine and mitomycin have also been associated with cardiotoxicity.
Other agents that may induce a cardiac event include paclitaxel, etoposide, teniposide, the vinca alkaloids, fluorouracil, cytarabine, amsacrine, cladribine, asparaginase, tretinoin and pentostatin.
Cardiotoxicity is rare with some agents, but may occur in >20% of patients treated with doxorubicin, daunorubicin or fluorouracil.
Cardiac events may include mild blood pressure changes, thrombosis, electrocardiographic changes, arrhythmias, myocarditis, pericarditis, myocardial infarction, cardiomyopathy, cardiac failure (left ventricular failure) and congestive heart failure.
These may occur during or shortly after treatment, within days or weeks after treatment, or may not be apparent until months, and sometimes years, after completion of chemotherapy.
A number of risk factors may predispose a patient to cardiotoxicity. These are:
.gif) |
cumulative dose (anthracyclines, mitomycin);
|
.gif) |
total dose administered during a day or a course (cyclophosphamide, ifosfamide, carmustine, fluorouracil, cytarabine);
|
.gif) |
rate of administration (anthracyclines, fluorouracil);
|
.gif) |
schedule of administration (anthracyclines);
|
.gif) |
mediastinal radiation; age;
|
.gif) |
female gender;
|
.gif) |
concurrent administration of cardiotoxic agents;
|
.gif) |
prior anthracycline chemotherapy;
|
.gif) |
history of or pre-existing cardiovascular disorders; and
|
.gif) |
electrolyte imbalances such as hypokalaemia and hypomagnesaemia.
|
The potential for cardiotoxicity should be recognized before therapy is initiated.
Patients should be screened for risk factors, and an attempt to modify them should be made.
Monitoring for cardiac events and their treatment will usually depend on the signs and symptoms anticipated and exhibited. Patients may be asymptomatic, with the only manifestation being electrocardiographic changes.
Continuous cardiac monitoring, baseline and regular electrocardiographic and echocardiographic studies, radionuclide angiography and measurement of serum electrolytes and cardiac enzymes may be considered in patients with risk factors or those with a history of cardiotoxicity.
Treatment of most cardiac events induced by chemotherapy is symptomatic. Agents that can be used prophylactically are few, although dexrazoxane, a cardioprotective agent specific for anthracycline chemotherapy, has been approved by the US Food and Drug Administration.
Cardiotoxicity can be prevented by
.gif) |
screening and modifying risk factors,
|
.gif) |
aggressively monitoring for signs and symptoms as chemotherapy is administered, and
|
.gif) |
continuing follow-up after completion of a course or the entire treatment.
|
Prompt measures such as discontinuation or modification of chemotherapy or use of appropriate drug therapy should be initiated on the basis of changes in monitoring parameters before the patient exhibits signs and symptoms of cardiotoxicity.
Resources
.gif) |
Cardiotoxicity and Cardiomyopathy - Managing Side Effects - Chemocare http://bit.ly/1vkSXlo
Note: the number of cycles of doxorubicin (the H in CHOP) is limited to six, which substantially reduces the risk of this side effect.
The above excellent resource has the following subtopics.
- What are cardiotoxicity and cardiomyopathy?
- Cardiomyopathy may be result of ...
- You may have developed cardiomyopathy if your doctor finds
- What are some symptoms to look for?
- Things you can do
- Drugs that may be prescribed by your doctor
- When to call your doctor or health care provider
Medications - such as certain types of chemotherapy may lead to cardiomyopathy. Medications that may commonly cause cardiotoxicity, or cardiomyopathy, are called anthracyclines. A commonly used anthracycline is called doxorubicin (Adriamycin®).
|
.gif) |
Cardiotoxicity of Anticancer Drugs: The Need for Cardio-Oncology and Cardio-Oncological Prevention ncbi.nlm.nih.gov
|
.gif) |
Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomized controlled trials ncbi.nlm.nih.gov
"Evidence is not sufficiently robust to support clear evidence-based recommendations on different anthracycline treatment regimens, or for routine use of cardiac protective agents or liposomal formulations. There is a need to improve cardiac monitoring in oncology trials."
|
.gif) |
Dexrazoxane for Preventing Anthracycline Cardiotoxicity in Children with Solid Tumors? ncbi.nlm.nih.gov
|
References and Research News
-
Valsartan May Prevent CHOP-Induced Cardiotoxicity Medscape (free login req.)
-
Nad(p)h oxidase and MRP genetic polymorphisms associate with doxorubicin-induced cardiotoxicity. ASCO 2004 Abstract No: 3021
-
Early cardiotoxicity of the CHOP regimen in aggressive non-Hodgkin's lymphoma.
Ann Oncol. 2003 Feb;14(2):277-81. PMID: 12562656
-
Safety and efficacy of pegylated liposomal doxorubicin (Doxil) in relapsed epithelial ovarian cancer patients: Effect of age and previous treatment. ASCO 2003 (3186)
-
Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood.
N Engl J Med. 1991 Mar 21;324(12):808-15. PMID: 1997853 | Related articles
-
Cardiac toxicity of alemtuzumab in patients with mycosis fungoides/Sezary syndrome.
Blood. 2004 Aug 1;104(3):655-8. Epub 2004 Apr 08. Review. PMID: 15073032 | Related articles
-
Pixantrone, A New Anthracycline with Possibly Less Cardiotoxicity cancerconsultants.com Jan 2005
-
Early cardiotoxicity of the CHOP regimen in aggressive non-Hodgkin's lymphoma.
Ann Oncol. 2003 Feb;14(2):277-81. PMID: 12562656
-
B-type natriuretic peptide (BNP) levels in patients receiving high-dose chemotherapy can detect those at risk of late cardio toxicity medscape.com (free login req.)
-
Patients with NHL Treated with Anthracyclines at Long-Term Risk for Chronic Heart Failure (2006)
According to an article recently published in the journal Blood, patients with aggressive non-Hodgkin’s lymphoma who receive six cycles of Adriamycin® (doxorubicin) have an increased risk of cardiovascular disease. These patients should undergo long-term monitoring for cardiac conditions. cancerconsultants.com
-
Anthracycline-Induced Cardiotoxicity http://bit.ly/aZOuVR
-
Chemotherapy and cardiac toxicity - the lesser of two evils doctorslounge.com
|
|