TOPICS
What are CAR T-cells? | Clinical Trials | Comments
TOPIC Search: PubMed | CART-specific | News and Reports
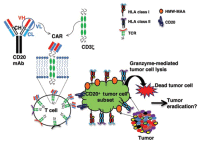 Chimeric Antigen Receptor (CAR) enhanced T-cells are lymphocytes enhanced to have cancer-killing ability. These cells with cancer fighting potential are taken from our body and then engineered to bind to a universally expressed protein on the tumor cell, such as cd19, but also cd20 and cd22 at this time. Chimeric Antigen Receptor (CAR) enhanced T-cells are lymphocytes enhanced to have cancer-killing ability. These cells with cancer fighting potential are taken from our body and then engineered to bind to a universally expressed protein on the tumor cell, such as cd19, but also cd20 and cd22 at this time.
CAR T-cells can persist and expand in the body where the cells act like "living drug" - combining the specificity of antibodies with the killing power of t-cells. Efficacy seems dependent on the ability of the enhanced cells to expand and persist in the body after infusion into the patients. More
Engineered T-cells in clinical-phase testing
.gif) |
Anti-Cd19 CAR T-cell therapy
(adoptive t-cell immunotherapy targeting cd19 on mature b-cells) Find trials
|
.gif) |
Chimeric antigen receptor (CARs) T-cells
(targeting cd19, cd20, cd22 (adoptive t-cell therapy) Find Trials
|
|
News and Reviews
.gif) |
Dec 2018 - Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. - PubMed - NCBI http://bit.ly/2GcI2sC
|
.gif) |
Clinical anti-lymphoma activity and toxicity of T cells expressing a novel anti-CD19 chimeric antigen receptor with fully-human variable regions. http://bit.ly/2yAREcL
|
.gif) |
Brewing T-cell therapies for leukemia and lymphoma - VJHemOnc http://bit.ly/2ASFmNc
|
.gif) |
About Fully human CD19-specific chimeric antigen receptors for T-cell therapy http://bit.ly/2gTbQi5
Trial: Testing T Cells Expressing a Fully-human AntiCD19 Chimeric Antigen Receptor for Treating B-cell Malignancies - reported to be near full accrual http://bit.ly/2xFmDzw
|
.gif) |
JCO 2017: Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels http://bit.ly/2ueiDH0 (full text)
Conclusion: "CAR-19 T cells preceded by low-dose chemotherapy induced remission of advanced-stage lymphoma, and high serum IL-15 levels were associated with the effectiveness of this treatment regimen. CAR-19 T cells will likely become an important treatment for patients with relapsed lymphoma.
interleukin-15 superagonist (IL-15SA) significantly enhances graft-versus-tumor activity. - PubMed - NCBI http://bit.ly/2tiCOk7
|
.gif) |
2017
CAR T-Cell Therapy KTE-C19 Appears Successful in Aggressive B-Cell Lymphoma - The ASCO Post http://bit.ly/2jLl6oJ
|
.gif) |
*Targeted Onc 2016:
KTE-C19 Demonstrates 52% Complete Remission Rate in Lymphoma Study http://bit.ly/2dJOrKw
|
.gif) |
New study reveals CAR T cells
can function as micro-pharmacies for precise therapeutic delivery http://bit.ly/2dnV9FO
|
.gif) |
Anti-CD19 CAR T cells preceded by low-dose chemo to induce remissions of advanced lymphoma. | 2016 ASCO Annual http://bit.ly/1XVVzoa
snip: "Nineteen patients with various subtypes of diffuse large B-cell lymphoma (DLBCL) had the following responses: 8 CR, 5 PR, 2 SD, and 4 PD. One patient with mantle cell lymphoma obtained a CR. Two patients with follicular lymphoma both obtained CRs. Durations of response currently range from 1 to 20 months; 10 remissions are ongoing. All but 4 patients had either chemotherapy-refractory lymphoma or lymphoma that had relapsed after autologous stem cell transplant. The most prominent toxicities were various neurological toxicities. Other toxicities included fever and hypotension."
|
.gif) |
2016 Blood Journal
Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in CLL http://bit.ly/24QYmC7
|
.gif) |
2016: Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies -
Europe PMC Article - full text http://bit.ly/1TFgYML
Snip:
Chimeric antigen receptor T (CAR-T) cell therapy has produced promising results in clinical trials but has been challenged by the inability to control engineered cells once infused into the patient. Here we present a generalizable method of controlling CAR-T cells using peptide-engrafted antibody-based molecular switches that act as a bridge between the target cell and CAR-T cell. We show that switches specific for CD19 govern the activity, tissue-homing, cytokine release, and phenotype of switchable CAR-T cells in a dose-titratable manner using xenograft mouse models of B-cell leukemia. We expect that this method of tuning CAR-T cell responses will provide improved safety and versatility of CAR–T-cell therapy in the clinic.
|
.gif) |
|
.gif) |
Review article: Dec 2015:
Adoptive T-cell therapies for refractory/relapsed leukemia & lymphoma:
current strategies and recent advances -
Adoptive T-cell immunotherapy is revolutionizing the treatment of hematologic malignancies and offers hope to patients with relapsing disease after HSCT and have few other therapeutic options. While there have been numerous advancements thus far, significant limitations remain. Future directions of research will include optimizing the generation of modified T cells to enhance not only efficacy but also the cost and time of manufacturing so that they may be readily available. Current and future clinical trials will hopefully build upon current successes with the potential to render adoptive T-cell immunotherapies the standard of care for hematologic malignancies for patients with refractory or relapsed disease.
Remaining challenges -- the need to: reduce and mitigate toxicity; overcoming tumor immune evasion; overcoming t-cell exhaustion; improving persistence (how long the t-cells last) and how to complement adoptive therapy with other treatments given before, with, or after.
|
.gif) |
NEJM, Oct 2014
CART19 and B-cell aplasia - a surrogate for persistent engineered t-cells -- associated with response http://bit.ly/1wrjgHc
Normal and malignant b-cells are ablated by the engineered t-cells programmed to kill cells expressing cd19.
The persistence of CART19 cells (and activity against tumor cells) can be gauged by the persistence of b-cell depletion.
|
.gif) |
J Clin Onc 2014:
CART19 in Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies http://bit.ly/Y5MbDV
|
.gif) |
OncoTherapy Network
Patient-Tailored Immunotherapy Receives Breakthrough Designation From FDA | http://bit.ly/1nuxSDd
|
.gif) |
News Medical 2013:
New reports demonstrate the effectiveness of modified T cells in treating blood-borne cancers http://bit.ly/JabInc
|
.gif) |
* Helio 2013:
CTL019 therapy induced response in CLL, ALL http://bit.ly/18Bic8r
* Medscape: 2013:
Excitement Over CAR-Engineered T-cells in Leukemia and Lymphoma http://bit.ly/1bQRLOL
|
.gif) |
* NIH 2013:
CAR T-Cell Therapy: Engineering Patients’ Immune Cells to Treat Their Cancer http://1.usa.gov/IzIHkU
* Int J Hematol 2013:
Chimeric antigen receptors for the adoptive T cell therapy of hematologic malignancies. http://1.usa.gov/18wvwYD
* Medscape 2013:
Excitement Over CAR-Engineered T-cells in Leukemia and Lymphoma http://bit.ly/1bQRLOL
* Sacremento B 2013:
Penn Medicine Team Reports Findings from Research Study of First 59 Adult and Pediatric Leukemia Patients Who Received Investigational, Personalized Cellular Therapy CTL019: http://bit.ly/1bRG4aK
Researchers Unveil First Results in Adult ALL Patients: All Five Patients Had Complete Response to Therapy, Genetically Modified Cells Produce Long-Term Remissions, Persist in Patients' Bodies with Vaccine-Like Activity for More than Three Year
* Marketwatch 2013:
Novartis highlights research on investigational, personalized T cell therapy CTL019 in patients with forms of acute and chronic leukemia - trials http://on.mktw.net/1ksxz5c
- Data at ASH show increased scientific understanding of CTL019 and its potential role in the treatment of certain types of lymphocytic leukemia(1,2,3,4) -- Presentations include findings that 19 of 22 pediatric patients with acute lymphoblastic leukemia (ALL) (86%) experienced complete remissions(1) -- Novartis and Penn exclusive global collaboration to develop chimeric antigen receptor (CAR) technology is moving forward with the goal of expanding clinical
*NPR 2013
Gene Therapy Scores Big Wins Against Blood Cancers http://n.pr/1hGBrm0
|
.gif) |
The Promising Path for CAR Immunotherapy - OncologyTube 
|
.gif) |
ASH :2013
High-tech gene-therapy advances offer hope for patients with hard-to-treat blood disorders http://bit.ly/1btaXjO
|
.gif) |
ASH Paper, 2013:
Effective Treatment Of Chemo-Refractory DLBC Lymphoma With Anti-CD19 CAR T-cells http://bit.ly/1h7HnUX
|
.gif) |
The Promising Path for CAR Immunotherapy - OncologyTube http://bit.ly/1aEpxkm
|
.gif) |
AACR 2013: Dr. Carl H. June, who was named one of Fast Company’s Most Creative People of 2013, spoke at this year’s Annual Meeting. Watch the free webcast of his talk,
“Harnessing the Immune System to Treat Cancer: Personalized and Targeted T-Cell Therapy.” http://bit.ly/13FSOv6
|
What are Chimeric Antigen Receptor T-cells (CAR T-cells)?
.gif) |
ImedexCME video: David Porter: University of PA - CARs - A Living drug
Porter: presentation - CART Therapy for CLL - YouTube 
|
An antigen is any distinctive shape (such as a protein) that can be recognized by the immune system as a binding point - like a key that fits only one type of lock.
To make CAR T-cells cancer-fighting t-cells are first collected from the patient, then modified to recognize an antigen binding site on the cancer cells, and then put back into the patient.
Remarkably, once put back into the patient the "programmed" t-cells can expand in number and persist for a long time and are capable of destroying any cells that have the target antigen.
We might think of this exciting technology as a way to modify t-cells to work similar to monoclonal antibody therapy (such as Rituxan that binds to cd20) - but with more potency and persistence - using live cells that can persist in the body ... instead of antibodies (proteins) that have are active for a limited time.
A more technical description:
"Chimeric antigen receptors (CARs) usually combine the antigen binding site of a monoclonal antibody with the signal activating machinery of a T cell, freeing antigen recognition from major histocompatibility complex restriction and thus breaking one of the barriers to more widespread application of cellular therapy.
Similar to treatment strategies employing monoclonal antibodies, T cells expressing CARs are highly targeted, but additionally offer the potential benefits of active trafficking to tumor sites, in vivo expansion and long term persistence. Furthermore, gene transfer allows the introduction of countermeasures to tumor immune evasion and of safety mechanisms.
Source: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3107373/
Reports and Background
Search Euro PubMed
.gif) |
Axicabtagene Ciloleucel (Kite CAR t-cell therapy targeting cd19)
Shows Response Rate of 76% in Aggressive Lymphoma http://bit.ly/2mEjHx2
|
.gif) |
Advanced Clinical Cell Processing Technologies for Adoptive Memory T Cell Therapy
(2016 AAAS February 2016)) http://bit.ly/1Kt6thv
Media report: Memory cells enhance strategy for fighting blood cancers |
Science News http://bit.ly/1Kt6TV9
|
.gif) |
Chimeric Antigen Receptor (CAR) T-Cell Therapy | Leukemia and Lymphoma Society http://bit.ly/1Lx2Lyc
|
.gif) |
ASH: T Cells Expressing CD19-Specific Chimeric Antigen Receptors Inhibited By
Indoleamine 2,3-Dioxygenase in Tumors http://bit.ly/1y3crwK
Targeting a mechanism of resistance to CART19
|
.gif) |
Recommended
Medscape (free login req.) ...
CAR T Cells: A Look Under the Hood and Down the Road http://bit.ly/1wFSHMW
|
.gif) |
BIG NEWS - Phila Business Journal 2014:
Penn gets FDA 'breakthrough' designation for experimental cancer therapy http://bit.ly/1lPtk3N
|
Technical background and overview
.gif) |
Gene Therapy: Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19 http://1.usa.gov/1nNtvQI
"The patient's lymphoma underwent a dramatic regression, and B-cell precursors were selectively eliminated from the patient's bone marrow after infusion of anti–CD19-CAR-transduced T cells. Blood B cells were absent for at least 39 weeks after anti–CD19-CAR-transduced T-cell infusion despite prompt recovery of other blood cell counts. Consistent with eradication of B-lineage cells, serum immunoglobulins decreased to very low levels after treatment. The prolonged and selective elimination of B-lineage cells could not be attributed to the chemotherapy that the patient received and indicated antigen-specific eradication of B-lineage cells. Adoptive transfer of anti–CD19-CAR-expressing T cells is a promising new approach for treating B-cell malignancies."
|
.gif) |
Immunotherapy of Malignant Disease Using Chimeric Antigen Receptor Engrafted T Cells http://1.usa.gov/1l3OugS
SNIP from background:
Tumour immunotherapy is one of the oldest branches of clinical immunology and has a long but checkered history. The overriding goal is to deploy the multiplicity of available immune effector mechanisms against tumour cells, but not against healthy counterparts. Unfortunately however, several obstacles render this a very difficult goal. Although hundreds of so-called tumour antigens have been identified, these are generally derived from self and thus are poorly immunogenic.
Furthermore, tumours use several mechanisms to render themselves hostile to the initiation and propagation of immune attack. These immune subversive strategies include reduced expression of HLA molecules and target antigens coupled with the establishment of a microenvironment in which inhibitory cytokines and leukocytes abound (recently reviewed in [1]). Indeed, cancer cells can even dedifferentiate to evade detection in response to inflammatory cues provided by tumour-specific T cells [2]. Consequently, it is not surprising that attempts to harness tumour-specific T cells using a succession of vaccination-based approaches have not achieved striking success.
|
Clinical Reports and Discussions of Same
.gif) |
The ASCO Post Mounting Success in Trials of Genetically Engineered T Cells to Treat Leukemias and Lymphomas http://bit.ly/KXVaPV
SNIP: ‘Smart Bomb’ Approach: The promise of the “smart bomb” approach with this immunologic therapy has attracted industry to partner with some investigators and their institutions. For example, CAR-T cells developed at the National Cancer Institute have been licensed to Kite Pharmaceuticals, and CAR-T cells developed at the University of Pennsylvania have been licensed to Novartis. In fact, Novartis is building a manufacturing facility to produce these cells near the University of Pennsylvania campus. Most recently, Fred Hutchinson Cancer Research Center, Memorial Sloan-Kettering Cancer Center, and Seattle Children’s Research Institute have joined forces to launch Juno Therapeutics, a new biotechnology company that will develop CAR-T cells.
|
.gif) |
April 2013: Gene Transfer Therapy Is Producing Prolonged Remissions in Patients with Advanced Leukemia
A Conversation with David L. Porter, MD ascopost.com
|
.gif) |
Regarding Tumor Response to Modified Autologous T Cells NEJM
Complete responses in 2 of 3 patients were reported, and significant improvement
in a partial responder with advanced/refractory Chronic Lymphocytic Leukemia
|
.gif) |
Chimeric Antigen Receptor–Modified T Cells for Acute Lymphoid Leukemia http://www.nejm.org/doi/full/10.1056/NEJMoa1215134
|
.gif) |
Video: Brian Koffman interview with Dr. Wierda
Practical Advice on CAR-T Trials Youtube
|
Technical Background
.gif) |
Chimeric Antigen Receptor (CAR)-Engineered Lymphocytes for Cancer Therapy http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3107373/
"... chimeric antigen receptors have human leukocyte antigen (HLA)-unrestricted activity and can target non-protein antigens (Table I). This is important as many tumors adopt immune evasion strategies affecting MHC processing and presentation"
|
.gif) |
Immunotherapy for B-Cell Neoplasms using T Cells expressing Chimeric Antigen Receptors
http://1.usa.gov/YTtfAi
|
Clinical Trials
.gif) |
anti-CD-19 directed engineered t-cells (CART19) (updated query) |
Illustrations
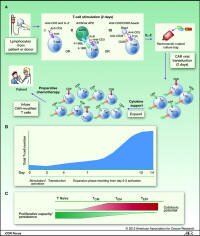
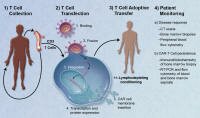
Is this a real breakthrough?
Yes, we feel that this report marks a genuine break-through in immunotherapy - made possible by amazing science (each scientist building on the insights of another) - but also by the patients willing to be the first-in-line to receive genetically engineered t-cells into their blood despite the risks and without prior evidence of efficacy.
Amazing science? Yes! In short, scientists modified T-cell taken from the patient so that the cells would kill all b-cells, malignant and normal - any cell with the CD19 receptor.
... Further, they made changes to the cells allowing the cells to expand and persist in the body, leading to the reversal of chemo-resistant CLL in the first few patients to have tried the new therapy.
How it was done is also noteworthy. The scientist "borrowed" a technique that HIV uses to infect cells - this time for good - in order to modify the t-cells so that they would behave in the desired way, but NOT to infect them with the HIV virus.
"For safety reasons lentiviral vectors never carry the genes required for their replication. To produce a lentivirus" ... wikipedia.org
Are there remaining questions?
Yes, there are many remaining questions and much work to do.
First, based on clinical experiences in only 3 patients with limited follow-up, we don’t know yet at what rate we will see this kind of response in other CLL or lymphoma patients, noting that it could prove effective for any b-cell lymphoma.
Second, we don't know if the complex techniques applied can generate effectively engineered t-cells consistently (each time in all patients) ... it may be that they can be, but we won't know for sure until it is verified by experience.
Further, we may yet learn that there are serious consequences from depleting all of our b-cells (our antibody-producing cells) – perhaps indefinitely – because of the long persistence of the activated t-cells.
(While treatment with Rituxan, which targets CD20 (a similar but different antigen) has a similar effect on normal b-cells, it is generally reversible with discontinuation of the drug.)
Understanding the potential risks requires also an understanding of the CD19 target:
"CD19 is expressed on follicular dendritic cells and B cells.
Source: miltenyibiotec pdf
There is more work to be done, and perhaps room for improvement. For example, scientists say that they should be able to turn off the activated t-cells with immune suppressing therapy if needed (and other means).
Might this technology make chemotherapy obsolete?
Probably not. It should be noted that this approach required pre-conditioning with chemotherapy to allow the "programmed-to-kill" t-cells to be accepted by the body and to allow them to multiply.
(The chemotherapy phase of this therapy reminds us of the "conditioning" therapy needed prior to the so-called mini allo stem cell rescue.)
What's next to do?
In a word, plenty, starting with the need to recruit many more patients for the studies ... to see if the reported results can be reproduced and at what rate, and also longer follow-up is needed to monitor the participants for possible delayed complications.
Is there a reason to be optimistic?
Yes, we think so, because in this case the clinical results were dramatic in patients with very advanced disease, and the regressions were causally linked to the activity of the engineered t-cells by findings from samples taken from the bone marrow.
Further, unlike chemical therapeutics, the programmed cells have no half-life limitation and the killing of malignant cells has very good (if not perfect) specificity - targeting mainly mature b-cells, a type of immune cell that one can live without (with IV immunoglobulin support) and that could regenerate once the programmed t-cells are disabled or eliminated.
Finally, we can hope that resistance (to this novel, living agent) might be futile - the cancer cells may not be able to escape or survive them. But ... again, we must note that there are many remaining questions, as described above.
Karl Schwartz
return to top
Snips from a NEJM article on this subject with comments within:
Chimeric ANTIGEN Receptor–Modified T Cells
Highly Active Against Chronic Lymphoid Leukemia
David L. Porter, M.D., Bruce L. Levine, Ph.D., Michael Kalos, Ph.D., Adam Bagg, M.D., and Carl H. June, M.D. August 10, 2011 (10.1056/NEJMoa1103849)
“We designed a lentiviral vector expressing a chimeric antigen receptor with specificity for the B-cell antigen CD19, coupled with CD137 (a costimulatory receptor in T cells [4-1BB]) and CD3-zeta (a signal-transduction component of the T-cell antigen receptor) signaling domains.
=> A clonal population of t-cell that is modified to seek out and destroy any cell that has the cd19 receptor – all mature b-cells, malignant and normal.
"A low dose (approximately 1.5×105 cells per kilogram of body weight) of autologous chimeric antigen receptor–modified T cells reinfused into a patient with refractory chronic lymphocytic leukemia (CLL) expanded to a level that was more than 1000 times as high as the initial engraftment level in vivo, with delayed development of the tumor lysis syndrome and with complete remission. "
=> One important aspect: The cells expanded in the body by 1000 times and led to a complete remission in patient with b-cell lymphoma/leukemia – chronic lymphocytic leukemia (CLL). This probably could not happen without preconditioning with chemotherapy.
"Apart from the tumor lysis syndrome, the only other grade 3/4 toxic effect related to chimeric antigen receptor T cells was lymphopenia."
=> This could be a major side effect, however. Leading to a deficiency in immunoglobulins and associated risk of chronic infection.
"Engineered cells persisted at high levels for 6 months in the blood and bone marrow and continued to express the chimeric antigen receptor."
=> This is both good and bad – good in that it could mean that the immune system will continue to eradicate any lymphoma/CLL cells for a long time – we don’t know yet how long. Bad in that the it could lead to long lasting immune deficiency.
A specific immune response was detected in the bone marrow, accompanied by loss of normal B cells and leukemia cells that express CD19. Remission was ongoing 10 months after treatment.
=> The immune response was confirmed by examination of bone marrow sample – so it is very likely caused by the adoptive t-cell treatment.
Hypogammaglobulinemia was an expected chronic toxic effect.
=> As noted above.
Link to further technical discussion in NEJM: http://www.nejm.org/doi/full/10.1056/NEJMe1106965
copying a snip:
“The tumor lysis syndrome was diagnosed 22 days after treatment and correlated temporally with the induction of high levels of cytokines (interferon-γ and interleukin-6) and with an increase in the number of circulating chimeric antigen receptor–positive T cells to a level that was nearly 1000 times as high as the level detected the day after infusion.
Eight months after therapy, chimeric antigen receptor–positive T cells persisted, and the patient had no evidence of disease on physical examination or on computed tomographic, flow-cytometric, or cytogenetic analysis. The expansion, persistence, and development of the memory phenotype, not to mention antitumor effects, of these T cells were impressive.”
Stand UP 2 Cancer-CRI Cancer IMMUNOTHERAPY
Research Dream Team Announced YouTube
|
Chimeric Antigen Receptor (CAR) enhanced T-cells are lymphocytes enhanced to have cancer-killing ability. These cells with cancer fighting potential are taken from our body and then engineered to bind to a universally expressed protein on the tumor cell, such as cd19, but also cd20 and cd22 at this time.

