We have discontinued our tax-exempt status
as of 2018
Lymphomation will remain non-profit,
however, and continue independently:
without funding from the healthcare industry.
Updates on our status will be posted here.
Thank you, so much for your support!
Education The topics we provide on lymphomation.org are based patient questions and needs. Our content is evidence-based, guided by our scientific advisors and the published medical literature.
Keeping Current We help patients to keep up with emerging approaches to treatment. We provide also commentary on the latest scientific reports - with a focus on levels of evidence. We also link the community to reputable sources of information on lymphoma topics.
Clinical Trials Informing patients about investigational therapies can be a critical way to provide support for lymphoma patients - such as when standard therapy is no longer effective. We provide education on investigational agents, reports, and tips on how to discuss trials with our doctors and second-opinion experts.
... We provide patient-friendly tools to help the community to locate trials that may be appropriate for the patient's type of lymphoma, treatment status, or to find trials based on the type of study drug.
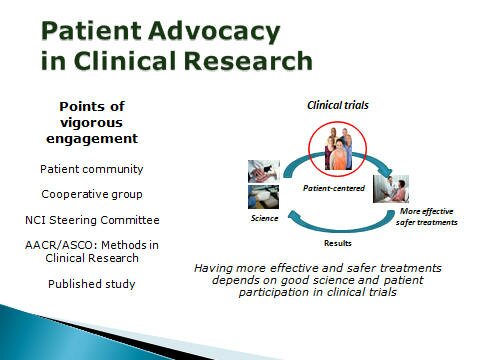
Research Advocacy We are liaisons with the research and patient communities -- helping patients to understand the science and scientists to better understand patients – the primary stakeholders in clinical research.
Research Advocacy Making additional Progress Against Lymphoma is urgently needed and requires the participation of patients - cannot be done without our consent to participate in studies or to give tissue and blood for correlative scientific research.
PAL engages the research community - providing patient perspectives on the direction of clinical research and the design of clinical trials. We advocate for clinical research that is patient-centered - focused on answering critical research questions ... but also having the potential to meet the clinical needs of the study participants.
Proudly serving as patient representative: Alliance Cooperative Group, Lymphoma Committee, FDA Advisory Committee, NCI: Progress Review Group, -Biospecimen Best Practice Workshops, -Steering Committee - Lymphoma, -Centralized Institutional Review Board, Patient Advocate Faculty ASCO/AACR Workshop: Methods in Clinical Cancer Research, Stand Up to Cancer, Joint Scientific Advisory Committee
To help make progress study designs must achieve two goals:
Good Science (providing reliable answers to important clinical questions)
AND Good Medicine (meeting the needs of the study participants)
We are the primary stakeholders in clinical research as it is the patient that must endure the disease and the limitations of existing therapies. (Not the shareholders, the researchers, or the clinical investigators.)
Helping patients to understand the science
and scientists to better understand patients –
the primary stakeholders in clinical research
Finally on a personal note: my spouse would not be here today (taking care of me and enjoying her life) if not for a new drug used in a novel way – a new drug made possible by excellent science but also by the patients willing to participate in the studies. The studies were essential -- to identify how to use the new agent safely and to test its efficacy. To focus on the plausible we had to learn (in our case the hard way) how to recognize suspect claims and theories – the claims made for alternative medicine for cancer.

Karl Schwartz, former president
and co-founder
Founder and former
President's CV
Lymphomation
Providing education, support, and research advocacy --
independent of industry funding - entirely dependent on public support
Lymphomation.org web stats - 2012
___
|