| In the News | IPI-145 | Idelalisib
PubMed Topic Search
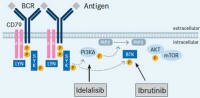 The so-called PI 3-kinases are signaling proteins within the cell that influence how the cells behave - their growth, persistence, and how they move (traffic) in the body. The so-called PI 3-kinases are signaling proteins within the cell that influence how the cells behave - their growth, persistence, and how they move (traffic) in the body.
Drugs designed to bind to (and inhibit) PI 3-kinases have promise as therapies for lymphoma and CLL.
Two such agents are in clinical phase testing for lymphoma and CLL:
Idelalisib and IPI-145. See below for links to trials and reports.
See for a video explanation by Cell Signaling.com:  and Genentech: and Genentech: 
Clinical Trials testing PI 3-kinase inhibitors:
IPI-145
(PI3K)-delta and PI3K-gamma
|
Idelalisib
PI3k delta
|
All PI3k inhibitors
|
IPI-145
(B or T lymphoma)
Find trials >
Reports |
Idelalisib / GS-1101 / CAL101
(B cell lymphoma)
Find trials >
Reports |
Lymphoma OR CLL Trials
for PI 3-kinase OR IPI-145 -
ClinicalTrials.gov |
* NEW:
Comparing IPI-145 Versus Ofatumumab for Relapsed or Refractory CLL/SLL
ClinicalTrials.gov |
CLL / SLL relapsed < 36 months after last treatment with current need to treat
Comparing: Idelalisib With Bendamustine VS Rituxan VS Bendamustine and Rituxan
Location: 82 centers in many regions of US and abroad. Open study above for details.
|
|
| |
Follicular lymphoma relapsed - responded to prior Rituxan-based treatment
Testing: Idelalisib (GS-1101 / CAL101) WITH Lenalidomide AND WITH Rituxan
Location: Alliance (CALGB) study: will open in multiple centers in many regions of US. Open study for details.
|
|
| |
b-cell lymphoma, relapsed and refractory
Testing: GS-9973 (oral syk inhibitor) WITH Idelalisib (b-cell receptor kinase inhibitor)
Locations: multiple centers in US
|
|
| |
indolent b-cell lymphoma, relapsed, responded to last Rituxan therapy, no transformed
Comparing Idelalisib (GS-1101) With Bendamustine-Rituxan VS Bendamustine-Rituxan
Participants have a 2:1 chance of being assigned to Idelalisib-based treatment group
|
|
| |
b-cell NHL, many types, indolent, relapsed, not transformed
Testing: Idelalisib WITH Rituxan
|
|
Idelalisib (CAL101) in the News
Idelalisib is an oral drug that binds to and inhibits a pathway (named phosphoinositide-3 kinase or PI3K) which fosters the abnormal growth and persistence of some kinds of blood cells through internal signals within the cells. The pathway is primarily active in activated b-cells, which limits but does not completely eliminate off-target effects (side effects). See also About Idelalisib (NCI dictionary)
* Idelalisib (PI3k inhibitor), in relapsed refractory indolent Non-Hodgkins Lymphoma? [Blood. 2014] - PubMed - NCBI http://1.usa.gov/OtIQJm
Comment: Showing good activity in relapsed setting. Limitation: Single arm study; eight doses evaluated.
64 patients; median age 64 (32-91) years; 34 (53%) had ≥1 lymph nodes ≥5 cm; 37 (58%) had refractory disease with median of 4 (1-10) prior therapies.
Eight dose regimens of Idelalisib were evaluated; idelalisib was taken once or twice daily continuously at doses ranging from 50 to 350 mg. After 48 weeks, patients still benefitting (n=19; 30%) enrolled into an extension study.
Adverse events occurring in 20% or more patients (AEs, ≥3%) were diarrhea (36/8%), fatigue (36/3%), nausea (25/3%), rash (25/3%), pyrexia / fever (20/3%), chills (20/0%). Laboratory abnormalities included neutropenia (44/23%), anemia (31/5%), thrombocytopenia (25/11%), and serum transaminase elevations (48/25%).
Twelve (19%) patients discontinued therapy due to AEs. Idelalisib induced disease regression in 46/54 (85%) of evaluable patients achieving an overall response rate of 30/64 (47%), with 1 patient having a complete response (1.6%).
Median duration of response was 18.4 months, median progression-free survival was 7.6 months. Idelalisib is well tolerated and active in heavily pretreated, relapsed/refractory patients with iNHL.
* NEJM
Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia — http://bit.ly/1oRaATu
* NEJM
PI3Kδ Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma — http://bit.ly/1jJAyrl
The response rate was 57% (71 of 125 patients), with 6% meeting the criteria for a complete response. The median time to a response was 1.9 months, the median duration of response was 12.5 months, and the median progression-free survival was 11 months.
COMMENT: How enthusiastic we will be about this report depends on our expectations. If we are anticipating that a single agent will be a home run leading to very durable remissions in the most challenging of clinical circumstances ... then this report will be disappointing.
There is reason to be encouraged, despite what might seem modest results at first glance. The positives include:
These responses were achieved in a population that have lymphoma refractory to regular treatments. The study group included "125 patients with indolent non-Hodgkin's lymphomas who had not had a response to rituximab and an alkylating agent or had had a relapse within 6 months."
The side effects of this agent are relatively mild and of a different type ... increasing the likelihood that it can be combined with regular treatments for greater effect - without decreasing the dose of the new drug or the regular treatments.
* ASH Paper:
Mature Response Data From a Phase 2 Study Of PI3K-Delta Inhibitor Idelalisib In Patients With Double (Rituximab and Alkylating Agent)-Refractory Indolent B-Cell Non-Hodgkin Lymphoma (iNHL) http://bit.ly/1bKKzSB
Idelalisib was well tolerated, had an acceptable safety profile, and was highly effective in this double-refractory iNHL population with an ORR of 57%. The ORR was consistent across all subgroups, regardless of disease histology, number of prior regimens, refractoriness to bendamustine, or tumor bulk. With continued administration of idelalisib, responses were durable beyond one year, substantially exceeding the median DOR for the last prior therapy.
* Medscape 2013:
Idelalisib Raises Eyebrows in Lymphoma http://bit.ly/18MV1Vu
IPI-145
A PI 3K inhibitor in clinical phase testing
* Comparing IPI-145 Versus Ofatumumab for Relapsed or Refractory CLL/SLL (DUO) -
ClinicalTrials.gov http://1.usa.gov/1bRPeCn
* ASH paper: “Preliminary Safety and Efficacy Of IPI-145, a Potent Inhibitor Of Phosphoinositide-3-Kinase-δ,γ, for CLL http://bit.ly/IZHTp7
Resources
.gif) |
Coming soon
|
|