TOPIC Search: PubMed
The tumor microenvironment
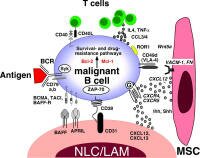 The tumor microenvironment (TME) is complex - it involves how neighboring bystander cells protect and promote lymphoma cells in the host environment typically within lymphoid organs and bone marrow. The tumor microenvironment (TME) is complex - it involves how neighboring bystander cells protect and promote lymphoma cells in the host environment typically within lymphoid organs and bone marrow.
We might think of this as the influence of a mob on the actions of bad individuals within the crowd - exciting and promoting bad behavior.
Importantly, there may be opportunities to block the promoting signals in order to treat the lymphoma - or the signals made by the lymphoma cells that suppress the immune response. See for example, PD-1/L targeting immune checkpoint blockade
Lymphomas appear to develop and thrive in the context of inflammatory and reactive conditions - sometimes auto-reactive - immune action against one's own cells. A long-festering infection, a chronic virus infection, h-pylori (in the case of MALT), an error (detecting self as non-self) etc. Reactive means acting against a threat. If one type of cell involved in this activity becomes a cancer it may still behave like a normal cell and require the complicity of the cells around it to sustain it's growth.
Here we will post articles that describe how non-malignant cells in the tumor microenvironment influence the clinical behavior of the disease; and how this may lead to new treatment strategies. We also provide TOPIC SEARCH queries so that you may easily locate additional articles on this topic as they are published.
 TOPIC SEARCH ~ FACTORS and SIGNALS in the MICROENVIRONMENT: TOPIC SEARCH ~ FACTORS and SIGNALS in the MICROENVIRONMENT:
New: PD-1/L targeting immune checkpoint blockade
angiogenesis and microvessel formation | PubMed | ASCO | Medscape
migration / homing, cell-to-cell adhesion | PubMed | ASCO | Medscape
cell survival and cell death signals | PubMed | ASCO | Medscape
antigen stimulation - follicular dendritic cells | PubMed | ASCO | Medscape
cell growth signals | PubMed
Immunity - infiltrating t-cells, macrophages, and NK cells | PubMed | Medscape
tumor escape and suppression of immunity | PubMed | Medscape | CTL-A4
Microenvironment ~ clinical course and response to therapies
Signaling with the microenvironment can promote the continuing survival and growth of tumor cells. The suppression of cell death (apoptosis) is one mechanism by which tumours become drug resistant. [16] These findings may explain the observation that Rituxan is often more effective on treating disease in the bone marrow than it is on nodal masses.
Surprisingly, the two gene-expression signatures that predicted survival, immune-response 1 and immune-response 2, comprised genes expressed by nonmalignant tumor-infiltrating cells. ... The statistical synergy of these two signatures in the survival model suggests that their relative contribution to the tumor's gene-expression profile - not their absolute expression levels - is of primary importance. In other words, the nature of the infiltrating immune cells was the predominant feature of the tumor that predicted the length of survival. [1]
The importance of host immunity: Recently, it was discovered that the profile of infiltrating t-cells in follicular lymphoma tumors, and not gene expression of the tumor cells, predicted how aggressive the lymphoma would be. Similarly, other reports show that immunity in the microenvironment predicts response to treatment and survival in follicular lymphomas. [1], [10]
"Predictive genes in two other signatures suggest that the host immune response has an important role in curative responses to chemotherapy. Expression of the lymph-node–signature genes reflects the non-tumor cells in the diffuse large-B-cell lymphoma–biopsy specimen, including activated macrophages, natural killer cells, and stromal cells. A high level of expression of these genes predicts a favorable clinical outcome, suggesting that this reactive immune response is beneficial. The MHC class II signature includes genes encoding components of this critical antigen-presentation–protein complex, and decreased expression of these genes predicts a poor outcome. These findings suggest that some tumors may evade the immune response by down-regulating their antigen-presentation capacity." [10]
Dysfunctional immune profiles (number and type) in the tumor microenvironment, can also lead to Tumour-Associated Macrophages (TAMS): disordered function, immune suppression and progressive tumour growth:
"Evidence currently available suggests that in established, progressively growing solid tumours, tumour associated macrophages (TAMs) are reprogrammed to induce immune suppression of host defenses in situ, through release of specific cytokines, prostanoids and other humoral mediators. This disordered response, results in the inhibition of effective anti-cancer cell-mediated immune mechanisms." [14]
The paradoxical observation that t-cells may sometimes stimulate b-cell proliferation:
In one study, purified follicular lymphoma cells underwent vigorous in vitro proliferation when cultured with a CD4+ T-cell clone that recognized an alloantigen expressed by the lymphoma cells. As seen in normal T-cell/B-cell interactions, the lymphoma cell proliferation was MHC class II dependent. In the absence of T-cells, the lymphoma cells did not respond to lymphokines [19]. This suggests that the T-cells commonly seen infiltrating follicular lymphomas may contribute to neoplastic B-cell proliferation. The paradoxical observation that T-cell infiltration is more commonly associated with spontaneous regression may indicate an early phase of the disease when the B-cells are still T-cell dependent and not autonomous. [24]
Predictive signatures came from immune cells:
" Two signatures--one which indicated poor prognosis, the other good--had strong synergy and together predicted survival better than any other model tested. Unexpectedly, both came from nonmalignant immune cells infiltrating the tumors. The good prognosis signature genes reflect a mixture of immune cells that is dominated by T cells. T cells react to specific threats to the body's health. In contrast, the poor prognosis signature genes reflect a different group of immune cells dominated by macrophages and/or dendritic cells--which react to nonspecific threats--rather than T cells. - http://www.cancer.gov/newscenter/pressreleases/FollicularLymphoma
Discussion: Like criminals, malignant cells arise from, and contribute to, dysfunctional environments. Understanding how lymphomas are suppressed or stimulated by host environments will undoubtedly lead to more sophisticated treatments, directed at the underlying causes that go beyond the tumor itself.
Interventions that may reverse the trend of diminishing response and response durations for indolent lymphomas may include: (1) Altering the host environment in ways that silence or inhibit pro-survival signals concurrent with standard therapies. (2) Focusing, and expanding immune surveillance against tumors. (3) Turning off signals and inhibiting cells that contribute to drug resistance, depressed immunity and escape from immunity. (4) Targeting tumors in ways that minimize damage to immunity, or enhance it. (5) Sequencing therapies in ways that restore positive elements of the microenvironment in order to consolidate the response to treatments.
Related Articles
-
Scientists Unlocking Lymphoma's Secrets - The microenvironment healthscout.com
"Genetic differences -- not in lymphoma cells, but in immune cells surrounding the tumor -- may determine how aggressive a particular case of follicular lymphoma turns out to be"
Prediction of Survival in Follicular Lymphoma Based on Molecular Features of Tumor-Infiltrating Immune Cells ~ NEJM November 18, 2004 vol. 351 no. 21 PMID 15548776 | Full text PDF (free registration req.)
-
T Helper Cell Activation in B-Cell Lymphomas jco.org
H. Bosshart University Hospital, Zurich, Switzerland
-
How do follicular dendritic cells interact intimately with B cells in the germinal centre?
Microenvironment connection to lymphoma:
"Follicular Dendritic cells (FDCs) prevent apoptosis of germinal centre B cells and stimulate cellular interaction and proliferation." ochsner.org PDF
"The germinal centre is a dynamic microenvironment where antigenactivated B cells rapidly expand and differentiate, generating plasma cells and memory B cells. These cellular events are accompanied by dramatic changes in the antibody molecules that undergo somatic hypermutation and isotype switching. Follicular dendritic cells (FDCs) are the stromal cells located in the germinal centre. Although the capacity of FDCs to present antigen to B cells through antigen–antibody complexes has been recognized for many years, additional critical functions of FDCs have only recently been recognized. FDCs prevent apoptosis of germinal centre B cells and stimulate cellular interaction and proliferation. Here, we review the FDC signaling molecules that have recently been identified, some of which offer potential therapeutic targets for autoimmune diseases and B-cell lymphomas."
-
The Role of the Tumor Microenvironment in Hematological Malignancies and Implication for Therapy bioscience.org
-
Staudt, Gene Expression Profiling of Lymphoid Malignancies - Annu.Rev.Med.2002.53:303–18 PubMed
-
CD4+ T-Cell Immune Response to Large B-Cell Non-Hodgkin’s Lymphoma Predicts Patient Outcome Stephen M. Ansell, Mary Stenson, Thomas M. Habermann, Diane F. Jelinek, Thomas E. Witzig JCO Feb 1 2001: 720-726. jco.org | PDF
-
Predicting Cancer Patient Survival with Gene Expression Data
DOI: 10.1371/journal.pbio.0020118 - full text: plosbiology.org
-
Bone Marrow Angiogenesis in Patients with Hematological Maligancies PDF
... stimuli in the bone marrow microenvironment, although macrophages ... this angiogenic
factor plays a role in bone
-
The indispensable role of microenvironment in the natural history of low-grade B-cell neoplasms Adv Cancer Res. 2000;79:157-73. Review. PMID: 10818680
-
Molecular Diagnosis of the Hematologic Cancers, Staudt NEJM May 1, 2003
-
Autologous Tumor Infiltrating T Cells Cytotoxic for Follicular Lymphoma Cells Can Be Expanded In Vitro By Joachim L. Schultze, Mark J. Seamon, Sabine Michalak, John G. Gribben, and Lee M. Nadler bloodjournal.org
-
CD40 ligand is constitutively expressed in a subset of T cell lymphomas and on the microenvironmental reactive T cells of follicular lymphomas and Hodgkin's disease ajp.amjpathol.org
-
Tumor escape: In Vivo Expression of B7-1 and B7-2 By Follicular Lymphoma Cells Can Prevent Induction of T-Cell Anergy But Is Insufficient to Induce Significant T-Cell Proliferation bloodjournal.org
-
Tumour-associated macrophages (TAMS): disordered function, immune suppression and progressive tumour growth B. AL-Sarireh and O. Eremin rcsed.ac.uk
-
Antagonistic effect of NK cells on alternatively activated monocytes: a contribution of NK cells to CTL generation bloodjournal.org
-
Survival signals within the tumour microenvironment suppress drug-induced apoptosis: lessons learned from B lymphomas. Endocr Relat Cancer. 1999 Mar;6(1):21-3. Review. PMID: 10732782
-
Follicular Pattern of Bone Marrow Involvement
from American Journal of Clinical Pathology Discussion Medscape (free login req.)
-
Retinoic acid inhibits the proliferative response induced by CD40 activation and interleukin-4 in mantle cell lymphoma. Cancer Res. 2005 Jan 15;65(2):587-95. PMID: 15695403
-
Adhesion and homing of tumor cells: The following might provide the basis for concurrent therapy: Standard therapy/antibodies with dugs that mobilize tumor cells, such as g-csf.
Quantification of CD4, CCR5, and CXCR$ levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages pubmedcentral.nih.gov
1 Homing and mobilization of hematopoietic stem cells and hematopoietic cancer cells are mirror image processes, utilizing similar signaling pathways and occurring concurrently: circulating cancer cells constitute an ideal target for concurrent treatment with chemotherapy and antilineage-specific antibodies
Y Gazitt1 1University of Texas Health Science Center, San Antonio, TX, USA Leukemia (2004) 18, 1–10 & 2004 Nature Publishing Group All rights reserved 0887-6924/04
(Homing of cancer cells in therapy-Gazitt.pdf)
2 A quantitative study of peripheral blood stem cell contamination in diffuse large-cell non-Hodgkin's lymphoma: one-half of patients significantly mobilize malignant cells
C. Jacquy,1 A. SoreÂe,1 F. Lambert,1 A. Bosly,2 A. Ferrant,2 M. AndreÂ,2 J. Parma,1 A. Kentos and P. Martiat1 1Free University of Brussels, Institut Jules Bordet and Hopital Erasme, Department of Haematology, Brussels, and 2Catholic University of Louvain, Groupe UCL d0HeÂmatologie, Brussels, Belgium Received 26 January 2000; accepted for publication 10 May 2000 ~ British Journal of Haematology, 2000, 110, 631±637
( CXCRGazittlymphomamobilisationGCSFBrJH2000.pdf)
3 Homeostatic Chemokines Drive Migration of Malignant B cells in Patients with Non-Hodgkin’s Lymphomas
Livio Trentin,* et al From the Department of Clinical and Experimental Medicine, Clinical Immunology Branch, Padua University School of Medicine, Venetian Institute for Molecular Medicine (VIMM), Centro di Eccellenza per la Ricerca Biomedica, 35128 Padova, Italy; Department of Experimental Biomedical Sciences, University of Padova, 35121 Padova, Italy. ~ Blood First Edition Paper, prepublished online March 4, 2004; DOI 10.1182/blood-2003-09-3103 (CXCR4C.pdf)
4 CXCR4 Neutralization, a Novel Therapeutic Approach for Non-Hodgkin’s Lymphoma
Francesco Bertolini et al Divisions of Hematology-Oncology [F. B., C. D., P. M., C. R., A. B., G. M.], Experimental Oncology-IFOM Institute of Molecular Oncology [S. M., A. G.], and Pathology [G. P.], European Institute of Oncology, 20141 Milan, Italy [CANCER RESEARCH 62, 3106–3112, June 1, 2002]
(CXCR4D.pdf)
5 Research Report Differential Mobilization of CD341 Cells and Lymphoma Cells in Non-Hodgkin’s Lymphoma Patients Mobilized with Different Growth Factors
YAIR GAZITT,1 PAUL SHAUGHNESSY,2 and QUN LIU1 JOURNAL OF HEMATOTHERAPY & STEM CELL RESEARCH 10:167–176 (2001) Mary Ann Liebert, Inc.
(CXCR4Gazittmobilisinglymphomacellswithcytokines.pdf)
-
Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood. 2005 Jun 2; PMID: 15933054
-
Tumour Microenvironment and Host Inflammatory Response Identified as Defining Features in Diffuse Large B-Cell Lymphoma: Presented at ICML docguide.com
-
Activation of Infiltrating Cytotoxic T Lymphocytes and Lymphoma Cell Apoptotic Rates in Gastric MALT Lymphomas Differences between High-Grade and Low-Grade Cases
Massimo Guidoboni*, Claudio Doglioni, Licia Laurino, Mauro Boiocchi* and Riccardo Dolcetti*
From the Division of Experimental Oncology 1,* amjpathol.org
-
Infectious disease associations in indolent lymphoma (follicular, FL and non-follicular, nFL): Developing a lymphoma prevention strategy. ASCO 2004
-
The Indolent Lymphomas
~ Ali W. Bseiso, MD, Peter McLaughlin, MD, Division of Medicine, The University of Texas M.D. Anderson Cancer Center, Houston, Texas cancernetwork.com/textbook
-
T Helper 2-Dominant Antilymphoma Immune Response Is Associated With Fatal Outcome
~ Peter P. Lee, Defu Zeng, Ann E. McCaulay, Yan-Fei Chen, Craig Geiler, Dale T. Umetsu, and Nelson J. Chao From the Department of Medicine, Divisions of Bone Marrow Transplantation and Immunology, and the Department of Pediatrics, Stanford University Medical Center, Stanford, CA. bloodjournal.org
-
Skewed T cell differentiation in indolent non-Hodgkin's lymphoma patients reversed by ex-vivo T cell culture with {gamma}c-cytokines. Blood. 2005 Sep 8; PMID: 16150945 | Related articles
-
Tumor microenvironment and mitotic checkpoint are key factors in the outcome of classical Hodgkin lymphoma. Blood. 2006 Mar 21; PMID: 16551964
-
Mantle cell lymphomas acquire increased expression of CCL4, CCL5 and 4-1BB-L implicated in cell survival. Int J Cancer. 2006 Apr 15;118(8):2092-7. PMID: 16287062
-
High numbers of tumor infiltrating FOXP3-positive regulatory T-cells are associated with improved overall survival in follicular lymphoma. Blood. 2006 Jul 6; PMID: 16825494
-
The Tumor Microenvironment Measured by Flow Cytometry Predicts Overall Survival (OS) and Transformation Risk (TR) in Follicular Lymphoma. Session Type: Poster Session, Board #584-II ASH 2006
"The proportion of CD8+ T cells relative to the total T cells and the number of residual, non-neoplastic B cells were both predictors of OS. Importantly, both predict, independently of the IPI, the risk of transformation. These biomarkers are easily measured and may be used to better stratify patients, choose initial treatment options and predict transformation risk in patients with FL."
-
Gene-Expression and Immunohistochemical Study of Specific T-Cell Subsets and Accessory Cell Types in the Transformation and Prognosis of Follicular Lymphoma. J Clin Oncol. 2007 Jan 2; PMID: 17200149 | Related articles
-
The microenvironment in mature B-cell malignancies: a target for new treatment strategies http://bit.ly/8TGgVc
- 2013, A microenvironment-mediated amplification loop in non-Hodgkin B cell lymphomas involving c-Myc/miR-548m/HDAC6 http://1.usa.gov/1m1fDnl
|