Lenalidomide is an Immune modulating drug (IMiD).
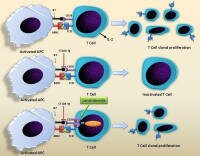 Lenalidomide is thought to alter the microenvironment in ways that favor activation of the immune system against the disease. Lenalidomide is thought to alter the microenvironment in ways that favor activation of the immune system against the disease.
The immune system is complex involving many types of special cells such as macrophages, dendritic cells, NK cells, T cells and B cells), and humoral components (antibodies, cytokines).
The immune system can prevent development of cancers by eliminating or suppressing oncogenic (cancer-driving) viral infections, altering the inflammatory factors that may support tumor growth, and by immune surveillance: identifying and destroying abnormal cells before they can cause harm.
Studies have shown that lenalidomide appears to work in different ways in different blood cell cancers. These mechanism involved direct cytotoxicity (killing of tumor cells without help from the immune system) as well as through indirect effects on tumor immunity -- helping the immune system to "see" or act on the tumor by changing the profile of host immune defenses.
How effective this drug can be may depend on the individual's immune status and the biology of the disease, which can vary by the type of lymphoma. Because its an oral drug with immune-modulating properties there is interest in testing lenalidomide with other immune-mediated drugs, such as Rituxan. ncbi.nlm.nih.gov
Lenalidomide has been shown to alter different parts of the immune system by changing cytokine production, regulating T cell co stimulation and augmenting the Natural Killer cell cytotoxicity.
The immune modulating properties of Lenalidomide are thought to account for its clinical efficacy in multiple myeloma, CLL and myelodysplastic syndromes; where the disease distorts the immune system by altering cytokine profiles in the tumor microenvironment.
Adapted from J Hematol Oncol. 2009 http://1.usa.gov/1a9Mi1a
In the News and Related Articles:
.gif) |
* Brit J Haematol 2014:
Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. http://1.usa.gov/Op7WsM
Comment: Encouraging to see good response rates in persons with Rituxan refractory lymphoma (n=13 at 61%) and a 44% CR rate overall. Limitations being the small study size (n =27) and no control group. One issue is that the protocol involves being on treatment for a long time (daily, indefinitely until toxicity or progression) and the impact that might have on quality of life … and also how to interpret duration of response when to achieve it requires continuous therapy. (Karl)
|
.gif) |
Business Wire 2013:
Studies Evaluating REVLIMID® (Lenalidomide) in Lymphoma Presented at ASH http://bit.ly/19goMBT
|
.gif) |
Immune-mediated actions of Lenalidomide
J Hematol Oncol. 2009; Mechanism of action of lenalidomide in hematological malignancies http://1.usa.gov/1a9Mi1a
|
.gif) |
J Hematol Oncol. 2013
Immunomodulation therapy with lenalidomide in follicular, transformed and diffuse large B cell lymphoma: current data on safety and efficacy http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3735404/
After the initial promising data from these small trials, a larger international phase II, single-arm, open-label study of lenalidomide in 217 patients with biopsy-proven relapsed or refractory aggressive DLBCL, FLG3, TmL, or MCL was initiated (NHL-003).
In the NHL-003 trial, 217 patients had received a median of 3 (range, 1–13) prior treatment regimens [8]. The ORR was 35%; the PR rate was 22% and the CR/CRu rate was 13%. The median PFS was 3.7 months, and the median follow-up period was 9.2 months. The most common grade 3 or 4 adverse events were neutropenia (41.0%), thrombocytopenia (19.0%), and anemia (9.2%).
In the NHL-003 trial, eight of the 19 patients with FLG3 (42%) achieved a response, two (11%) of whom achieved CR; the median PFS duration in this trial was 8.9 months.9
|
.gif) |
Br J Haematol. 2013
Lenalidomide efficacy in activated B-cell-like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. http://www.ncbi.nlm.nih.gov/pubmed/23252516
|
.gif) |
PubMed TOPIC Search: Rituxan AND Lenalidomide ncbi.nlm.nih.gov
|
.gif) |
Mechanism of action of lenalidomide in hematological malignancies http://1.usa.gov/1a9Mi1a
|
|